Revolutionizing Orthopedic Rehabilitation: How Exoskeleton Robotics Will Transform Patient Outcomes and Market Dynamics in 2025 and Beyond
- Executive Summary: 2025 Market Outlook and Key Drivers
- Current State of Exoskeleton Robotics in Orthopedic Rehabilitation
- Leading Companies and Recent Innovations (e.g., eksohealth.com, rewalk.com, suitx.com)
- Market Size, Segmentation, and 2025–2030 Growth Forecasts (Estimated CAGR: 18–22%)
- Technological Advancements: AI, Sensors, and Materials
- Clinical Efficacy and Patient Outcomes: Evidence and Case Studies
- Regulatory Landscape and Reimbursement Trends (e.g., fda.gov, aaos.org)
- Adoption Barriers: Cost, Training, and Integration in Healthcare Systems
- Emerging Applications and Future Opportunities (2025–2030)
- Strategic Recommendations and Future Outlook for Stakeholders
- Sources & References
Executive Summary: 2025 Market Outlook and Key Drivers
The exoskeleton robotics market for orthopedic rehabilitation is poised for significant growth in 2025, driven by technological advancements, increasing prevalence of musculoskeletal disorders, and expanding clinical adoption. Exoskeletons—wearable robotic devices designed to assist or enhance limb movement—are increasingly being integrated into rehabilitation protocols for patients recovering from orthopedic injuries, surgeries, or neurological conditions such as stroke and spinal cord injury.
Key industry players are accelerating innovation and commercialization. Ekso Bionics, a pioneer in medical exoskeletons, continues to expand its global footprint, with its EksoNR device being adopted by leading rehabilitation centers for gait training and mobility restoration. ReWalk Robotics is also advancing its exoskeleton solutions, focusing on both clinical and personal use, and has received regulatory clearances in multiple regions, supporting broader market access. CYBERDYNE Inc. from Japan is notable for its HAL (Hybrid Assistive Limb) exoskeleton, which is being utilized in hospitals and rehabilitation facilities across Asia and Europe, with ongoing clinical studies supporting its efficacy.
In 2025, the market is expected to benefit from several converging trends. The global burden of orthopedic conditions—driven by aging populations and rising incidence of trauma and degenerative diseases—continues to increase demand for effective rehabilitation solutions. Exoskeletons offer the potential for more intensive, repetitive, and personalized therapy, which can accelerate recovery and improve patient outcomes compared to conventional methods. Additionally, ongoing improvements in sensor technology, artificial intelligence, and lightweight materials are making exoskeletons more user-friendly and accessible.
Healthcare systems and insurers are beginning to recognize the value proposition of robotic rehabilitation, with some providers integrating exoskeletons into standard care pathways. Partnerships between device manufacturers and hospital networks are expanding, and new reimbursement models are being piloted in select markets. For example, Ottobock, a global leader in prosthetics and orthotics, has entered the exoskeleton space with its Paexo product line, targeting both industrial and medical applications.
Looking ahead, the exoskeleton robotics sector is expected to see continued investment and regulatory progress, with more devices gaining approvals and entering clinical use. The next few years will likely witness broader adoption in rehabilitation centers, increased home-use options, and further integration with digital health platforms. As clinical evidence accumulates and costs decrease, exoskeletons are positioned to become a standard component of orthopedic rehabilitation worldwide.
Current State of Exoskeleton Robotics in Orthopedic Rehabilitation
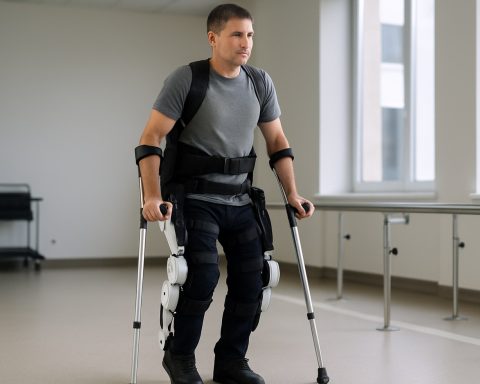
Exoskeleton robotics have rapidly advanced as a transformative technology in orthopedic rehabilitation, offering new hope for patients recovering from musculoskeletal injuries, surgeries, and neurological conditions. As of 2025, the sector is characterized by a growing number of clinically validated devices, increased adoption in rehabilitation centers, and ongoing innovation aimed at improving patient outcomes and accessibility.
Several leading manufacturers have established themselves as key players in the orthopedic exoskeleton market. Ekso Bionics is widely recognized for its EksoNR exoskeleton, which is FDA-cleared for use in rehabilitation of patients with acquired brain injury, stroke, and spinal cord injury. The device is now deployed in hundreds of rehabilitation facilities worldwide, with clinical studies demonstrating improved gait training and functional mobility for users. Similarly, ReWalk Robotics offers exoskeletons designed for both clinical and personal use, with its ReWalk Personal 6.0 system enabling individuals with lower limb disabilities to walk independently in their homes and communities.
European companies are also at the forefront of innovation. Ottobock, a global leader in prosthetics and orthotics, has expanded its exoskeleton portfolio with the Paexo series, targeting both industrial and medical applications. The company’s medical exoskeletons are increasingly integrated into orthopedic rehabilitation programs, particularly for upper limb support and post-surgical recovery. Meanwhile, Hocoma (a member of the DIH Group) continues to advance robotic gait training with its Lokomat system, which is widely used in hospitals and rehabilitation centers for intensive, repetitive locomotion therapy.
Recent years have seen a surge in clinical research and pilot programs evaluating the efficacy of exoskeleton-assisted rehabilitation. Data from multicenter trials indicate that robotic exoskeletons can accelerate recovery timelines, enhance patient engagement, and reduce therapist workload. For example, studies involving Ekso Bionics and Hocoma devices report significant improvements in walking speed, endurance, and balance among orthopedic patients.
Looking ahead, the outlook for exoskeleton robotics in orthopedic rehabilitation is highly promising. Industry leaders are investing in lighter, more adaptive devices with advanced sensor integration and AI-driven personalization. There is also a trend toward expanding access beyond specialized clinics, with portable and home-use exoskeletons under development. As regulatory pathways become clearer and reimbursement models evolve, adoption is expected to accelerate, making robotic exoskeletons an integral part of orthopedic rehabilitation protocols in the coming years.
Leading Companies and Recent Innovations (e.g., eksohealth.com, rewalk.com, suitx.com)
The exoskeleton robotics sector for orthopedic rehabilitation is experiencing rapid growth and technological advancement, with several leading companies driving innovation and clinical adoption. As of 2025, the market is characterized by a focus on improving patient mobility, reducing rehabilitation times, and integrating smart technologies for personalized therapy.
One of the most prominent players is Ekso Bionics, known for its EksoNR exoskeleton, which is FDA-cleared for use in rehabilitation of patients with acquired brain injury, stroke, and spinal cord injury. In recent years, Ekso Bionics has expanded its clinical partnerships and enhanced its device with adaptive software that provides real-time feedback to therapists and patients, aiming to optimize gait training outcomes. The company continues to collaborate with major rehabilitation centers globally, supporting large-scale clinical studies and data collection to further validate the efficacy of robotic exoskeletons in orthopedic recovery.
Another key innovator is ReWalk Robotics, which offers both personal and rehabilitation exoskeleton systems. The ReWalk Personal 6.0 system is designed for everyday use by individuals with lower limb disabilities, while the ReStore Exo-Suit targets post-stroke gait training. In 2024 and 2025, ReWalk has focused on expanding insurance coverage and regulatory approvals in new markets, as well as integrating cloud-based analytics to track patient progress and tailor therapy regimens. The company’s ongoing research collaborations with hospitals and universities are expected to yield further improvements in device ergonomics and user experience.
California-based SuitX (now part of Ottobock), has continued to develop modular exoskeletons such as the Phoenix, which is lightweight and designed for both clinical and personal use. SuitX’s approach emphasizes affordability and adaptability, making its devices accessible to a broader range of rehabilitation centers and patients. Since its acquisition by Ottobock, a global leader in prosthetics and orthotics, SuitX has benefited from expanded distribution channels and increased R&D resources, accelerating the pace of innovation and clinical adoption.
Other notable contributors include CYBERDYNE Inc., whose HAL (Hybrid Assistive Limb) exoskeleton is widely used in Japan and Europe for neurorehabilitation, and Hocoma, which integrates robotic exoskeletons with advanced therapy software for comprehensive rehabilitation solutions.
Looking ahead, the next few years are expected to see further integration of artificial intelligence, improved sensor technologies, and greater interoperability with telemedicine platforms. These advancements will likely enhance the precision and accessibility of exoskeleton-assisted orthopedic rehabilitation, supporting broader adoption in both clinical and home settings.
Market Size, Segmentation, and 2025–2030 Growth Forecasts (Estimated CAGR: 18–22%)
The global market for exoskeleton robotics in orthopedic rehabilitation is poised for robust expansion between 2025 and 2030, with an estimated compound annual growth rate (CAGR) ranging from 18% to 22%. This growth is driven by increasing incidences of musculoskeletal disorders, a rapidly aging population, and technological advancements that are making exoskeletons more accessible and effective for clinical and home-based rehabilitation.
Market segmentation reveals that the healthcare sector—particularly orthopedic and neurological rehabilitation—remains the dominant application area. Within this, lower limb exoskeletons account for the largest share, as they address mobility impairments resulting from stroke, spinal cord injuries, and degenerative diseases. Upper limb exoskeletons are also gaining traction, especially for post-surgical recovery and rehabilitation of arm and shoulder injuries.
Regionally, North America and Europe are expected to maintain leadership due to established healthcare infrastructure, favorable reimbursement policies, and early adoption of advanced rehabilitation technologies. The United States, in particular, is home to several pioneering companies and research institutions. Asia-Pacific is projected to witness the fastest growth, fueled by rising healthcare investments, increasing awareness, and government initiatives to modernize rehabilitation services.
Key industry players are actively expanding their portfolios and global reach. ReWalk Robotics is a prominent manufacturer specializing in wearable robotic exoskeletons for lower limb rehabilitation, with FDA-cleared devices for both clinical and personal use. Ekso Bionics offers exoskeletons designed for neurorehabilitation and industrial applications, and has established partnerships with leading rehabilitation centers worldwide. CYBERDYNE Inc. from Japan is recognized for its Hybrid Assistive Limb (HAL) exoskeleton, which leverages bioelectric signals to support voluntary movement in patients with mobility impairments. Hocoma, a Swiss company, provides robotic rehabilitation solutions including the Lokomat, a widely used gait training exoskeleton in clinical settings.
The outlook for 2025–2030 is characterized by ongoing innovation, with companies focusing on lighter, more ergonomic designs, improved sensor integration, and AI-driven adaptive control systems. Integration with tele-rehabilitation platforms and data analytics is expected to enhance personalized therapy and remote monitoring. As regulatory pathways become clearer and costs decrease, adoption is anticipated to accelerate not only in hospitals and rehabilitation centers but also in outpatient and home environments, broadening the market’s reach and impact.
Technological Advancements: AI, Sensors, and Materials
The field of exoskeleton robotics for orthopedic rehabilitation is experiencing rapid technological advancements, particularly in the integration of artificial intelligence (AI), advanced sensor systems, and novel materials. These innovations are shaping the next generation of wearable robotic devices, with significant implications for patient outcomes and clinical adoption in 2025 and the coming years.
AI-driven control systems are at the forefront of this evolution. Modern exoskeletons increasingly utilize machine learning algorithms to interpret user intent, adapt to individual gait patterns, and optimize assistance in real time. For example, ReWalk Robotics and Ekso Bionics are both incorporating AI-based adaptive control to enhance user safety and personalize rehabilitation protocols. These systems can analyze biomechanical data from embedded sensors, enabling the exoskeleton to respond dynamically to changes in movement or muscle activity.
Sensor technology is another critical area of advancement. The latest exoskeletons are equipped with a suite of sensors—including inertial measurement units (IMUs), force sensors, and electromyography (EMG) sensors—that provide high-resolution feedback on joint angles, muscle activation, and load distribution. Companies like CYBERDYNE Inc. have pioneered the use of bioelectrical signal detection, allowing their devices to initiate movement based on the user’s voluntary muscle signals. This approach not only improves the naturalness of assisted movement but also supports more effective neuromuscular retraining during rehabilitation.
Material science is also playing a pivotal role in the evolution of exoskeletons. The adoption of lightweight, high-strength materials such as carbon fiber composites and advanced polymers is making devices more comfortable and less fatiguing for users. Hocoma, a leader in rehabilitation robotics, has focused on ergonomic design and material innovation to ensure their exoskeletons are suitable for extended clinical use. These material improvements are crucial for increasing patient compliance and expanding the range of orthopedic conditions that can be addressed.
Looking ahead, the convergence of AI, sensor fusion, and advanced materials is expected to yield exoskeletons that are more intuitive, adaptable, and accessible. Industry leaders are investing in cloud-based data analytics and remote monitoring, which will further personalize therapy and enable large-scale outcome tracking. As regulatory pathways become clearer and clinical evidence mounts, the adoption of these next-generation exoskeletons in orthopedic rehabilitation is poised to accelerate through 2025 and beyond.
Clinical Efficacy and Patient Outcomes: Evidence and Case Studies
The clinical efficacy of exoskeleton robotics in orthopedic rehabilitation continues to be a focal point of research and deployment in 2025, with a growing body of evidence supporting their role in improving patient outcomes. Exoskeletons are being increasingly integrated into rehabilitation protocols for conditions such as stroke, spinal cord injury, and post-operative orthopedic recovery, with several leading manufacturers and healthcare institutions reporting promising results.
Recent multicenter clinical trials and case studies have demonstrated that robotic exoskeletons can significantly enhance gait training, muscle strength, and functional independence in patients with lower limb impairments. For example, the Ekso Bionics EksoNR exoskeleton has been utilized in rehabilitation centers worldwide, with published data indicating improvements in walking speed, endurance, and balance among stroke and spinal cord injury patients. Similarly, ReWalk Robotics has reported that its ReWalk Personal 6.0 system enables individuals with lower limb paralysis to achieve upright mobility, with clinical studies showing increased community ambulation and reduced secondary complications such as pressure sores and muscle atrophy.
In orthopedic rehabilitation specifically, exoskeletons are being used to accelerate recovery following joint replacement surgeries and traumatic injuries. The CYBERDYNE Inc. HAL (Hybrid Assistive Limb) system, for instance, has been adopted in Japanese and European clinics for post-operative gait training, with case reports highlighting faster restoration of walking ability and reduced rehabilitation times compared to conventional therapy. Additionally, BIONIK Laboratories has expanded the deployment of its InMotion ARM and InMotion LEG robots, with ongoing studies in 2025 focusing on upper and lower limb rehabilitation for orthopedic patients.
Patient-reported outcomes are also a key metric, with surveys and qualitative studies indicating high satisfaction rates due to the increased sense of autonomy and motivation provided by exoskeleton-assisted therapy. Importantly, safety profiles remain favorable, with adverse events being rare and typically limited to minor skin irritation or device fit issues.
Looking ahead, the outlook for exoskeleton robotics in orthopedic rehabilitation is optimistic. Ongoing advancements in sensor technology, artificial intelligence, and device ergonomics are expected to further personalize therapy and improve outcomes. As regulatory approvals expand and reimbursement pathways become clearer, adoption is projected to increase across hospitals and outpatient clinics globally. The next few years are likely to see more robust, long-term data from randomized controlled trials, further cementing the role of exoskeletons as a standard adjunct in orthopedic rehabilitation.
Regulatory Landscape and Reimbursement Trends (e.g., fda.gov, aaos.org)
The regulatory landscape for exoskeleton robotics in orthopedic rehabilitation is evolving rapidly as these devices transition from research prototypes to clinically validated tools. In the United States, the U.S. Food and Drug Administration (FDA) classifies most lower-limb exoskeletons as Class II medical devices, requiring 510(k) premarket notification. Over the past few years, several exoskeletons have received FDA clearance for rehabilitation use, including devices from Ekso Bionics and ReWalk Robotics. These clearances are based on demonstrated safety and effectiveness in assisting patients with mobility impairments due to stroke, spinal cord injury, or other orthopedic conditions.
In 2025, regulatory agencies are expected to further refine guidance for exoskeletons, particularly as new models incorporate advanced sensors, AI-driven gait analysis, and remote monitoring features. The FDA has signaled interest in developing specific guidelines for robotic rehabilitation devices, focusing on cybersecurity, interoperability, and real-world performance data. The European Union’s Medical Device Regulation (MDR) also imposes stricter requirements for clinical evidence and post-market surveillance, impacting manufacturers such as CYBERDYNE Inc. and Hocoma AG, both of which have a significant presence in the European market.
Reimbursement remains a critical challenge for widespread adoption. In the U.S., the Centers for Medicare & Medicaid Services (CMS) has yet to establish a dedicated reimbursement code for exoskeleton-assisted therapy, though some private insurers have begun to cover sessions on a case-by-case basis. Advocacy by organizations such as the American Academy of Orthopaedic Surgeons (AAOS) and device manufacturers is ongoing, with efforts focused on demonstrating cost-effectiveness and improved patient outcomes. In 2024 and 2025, several large-scale clinical trials are underway to provide the robust data needed for broader insurance coverage.
Looking ahead, the regulatory and reimbursement environment is expected to become more favorable as evidence accumulates and as exoskeletons become more integrated into standard orthopedic rehabilitation protocols. Industry leaders like Ekso Bionics, ReWalk Robotics, and CYBERDYNE Inc. are actively engaging with regulators and payers to shape policy and facilitate access. The next few years will likely see increased harmonization of standards across major markets, paving the way for broader clinical adoption and improved patient access to exoskeleton-assisted rehabilitation.
Adoption Barriers: Cost, Training, and Integration in Healthcare Systems
The adoption of exoskeleton robotics for orthopedic rehabilitation is accelerating, yet significant barriers remain in 2025, particularly regarding cost, clinical training, and integration into existing healthcare systems. These challenges are shaping the pace and scale at which exoskeletons are deployed in hospitals, rehabilitation centers, and outpatient clinics.
Cost remains a primary obstacle. Advanced exoskeletons, such as those developed by Ekso Bionics, ReWalk Robotics, and CYBERDYNE Inc., can cost between $70,000 and $150,000 per unit, excluding maintenance and software updates. While some manufacturers are working to reduce prices through modular designs and leasing models, the initial investment is still prohibitive for many smaller clinics and rehabilitation centers. Reimbursement policies are evolving, but in most regions, insurance coverage for exoskeleton-assisted therapy remains limited, further constraining widespread adoption.
Training is another significant barrier. Effective use of exoskeletons requires specialized training for physical therapists and clinical staff. Companies like Ekso Bionics and ReWalk Robotics offer certification programs and on-site training, but the learning curve can be steep, especially for facilities with high staff turnover or limited experience with robotic technologies. In 2025, some healthcare systems are beginning to integrate exoskeleton training into broader rehabilitation curricula, but standardized protocols are still lacking, and ongoing education is necessary to keep pace with rapid technological advancements.
Integration into healthcare systems presents both technical and organizational challenges. Exoskeletons must be compatible with existing electronic health record (EHR) systems and rehabilitation workflows. Interoperability is a focus for leading manufacturers, with CYBERDYNE Inc. and Ekso Bionics developing software interfaces to facilitate data sharing and outcome tracking. However, integration often requires significant IT investment and workflow redesign, which can delay implementation. Additionally, regulatory requirements and safety standards are evolving, with organizations such as the International Organization for Standardization (ISO) and national health authorities updating guidelines for robotic medical devices.
Looking ahead, the outlook for exoskeleton adoption in orthopedic rehabilitation is cautiously optimistic. As costs gradually decrease, training resources expand, and integration becomes more seamless, adoption rates are expected to rise, particularly in large hospital networks and specialized rehabilitation centers. However, overcoming these barriers will require continued collaboration between manufacturers, healthcare providers, and policymakers to ensure that exoskeleton technology can be deployed safely, effectively, and equitably.
Emerging Applications and Future Opportunities (2025–2030)
Exoskeleton robotics for orthopedic rehabilitation is poised for significant expansion and innovation between 2025 and 2030, driven by advances in sensor technology, artificial intelligence, and materials science. These wearable robotic devices, designed to assist or augment human movement, are increasingly being integrated into clinical rehabilitation programs for patients recovering from orthopedic injuries, surgeries, or neurological conditions affecting mobility.
A key trend is the transition from hospital-based exoskeleton use to broader adoption in outpatient clinics and even home settings. Companies such as Ekso Bionics and ReWalk Robotics are at the forefront, offering FDA-cleared exoskeletons for lower limb rehabilitation. Their devices are being used to help patients regain walking ability after spinal cord injuries or strokes, with clinical studies demonstrating improvements in gait, balance, and muscle strength. In 2025, these companies are expected to further refine their products for greater comfort, adaptability, and ease of use, targeting not only hospitals but also physical therapy centers and home-based care.
Another emerging application is the integration of real-time data analytics and machine learning to personalize rehabilitation protocols. Exoskeletons equipped with advanced sensors can collect detailed biomechanical data, enabling therapists to tailor therapy sessions to individual patient needs. SuitX, now part of Ottobock, is developing modular exoskeletons that can be adjusted for different joints and rehabilitation stages, supporting a more customized approach to recovery.
Pediatric orthopedic rehabilitation is also gaining attention, with companies like NextStep Robotics and Bionik Laboratories exploring lightweight, adjustable exoskeletons for children with cerebral palsy or developmental disorders. These innovations aim to improve mobility outcomes and quality of life for younger patients, a segment previously underserved by traditional exoskeleton designs.
Looking ahead, the next five years are likely to see increased collaboration between exoskeleton manufacturers, healthcare providers, and insurers to establish standardized protocols and reimbursement models. The growing body of clinical evidence supporting exoskeleton-assisted rehabilitation is expected to drive wider acceptance and integration into mainstream orthopedic care. Additionally, advances in soft robotics and lightweight materials may lead to more affordable and user-friendly devices, expanding access to a broader patient population.
Overall, the period from 2025 to 2030 is set to witness exoskeleton robotics becoming a cornerstone of orthopedic rehabilitation, with ongoing innovation promising improved patient outcomes, greater independence, and reduced healthcare costs.
Strategic Recommendations and Future Outlook for Stakeholders
The exoskeleton robotics sector for orthopedic rehabilitation is poised for significant evolution in 2025 and the coming years, driven by technological advancements, regulatory developments, and increasing clinical adoption. Stakeholders—including manufacturers, healthcare providers, payers, and policymakers—should consider several strategic recommendations to maximize opportunities and address emerging challenges.
1. Prioritize Clinical Validation and Real-World Evidence
Robust clinical validation remains critical for widespread adoption. Companies such as Ekso Bionics and ReWalk Robotics have invested in clinical trials to demonstrate efficacy in post-stroke and spinal cord injury rehabilitation. Stakeholders should continue to support and publish peer-reviewed studies, focusing on long-term outcomes, cost-effectiveness, and patient quality of life. Collaborations with leading rehabilitation centers can accelerate data collection and strengthen the evidence base.
2. Expand Indications and Personalization
While early exoskeletons targeted spinal cord injuries, recent years have seen expansion into stroke, multiple sclerosis, and elderly mobility. Companies like CYBERDYNE Inc. are developing modular systems adaptable to various patient needs. Stakeholders should invest in R&D for personalized, adjustable devices, leveraging AI and sensor integration to tailor therapy intensity and support to individual recovery trajectories.
3. Address Reimbursement and Regulatory Pathways
Securing reimbursement is a major hurdle. In 2024, ReWalk Robotics achieved a key milestone with U.S. Medicare coverage for its exoskeletons, setting a precedent for others. Stakeholders should engage proactively with payers and regulatory bodies to clarify pathways, develop health economic models, and advocate for broader coverage, especially as new clinical data emerges.
4. Foster Integration with Digital Health Ecosystems
Integration with tele-rehabilitation platforms and remote monitoring tools is increasingly important. Companies such as Hocoma are incorporating cloud-based analytics and remote progress tracking. Stakeholders should prioritize interoperability, data security, and user-friendly interfaces to enhance patient engagement and enable hybrid care models.
5. Prepare for Market Expansion and Globalization
The Asia-Pacific region, led by Japan and China, is expected to see rapid growth due to aging populations and supportive government initiatives. CYBERDYNE Inc. and SUITX (now part of Ottobock) are expanding their international presence. Stakeholders should adapt products to local regulatory requirements, languages, and clinical practices, and consider strategic partnerships for distribution and service.
Outlook: By 2025 and beyond, exoskeleton robotics for orthopedic rehabilitation will likely become more accessible, affordable, and clinically integrated. Stakeholders who invest in evidence generation, regulatory engagement, digital integration, and global market strategies will be best positioned to lead in this dynamic sector.
Sources & References
- ReWalk Robotics
- CYBERDYNE Inc.
- Ottobock
- Hocoma
- ReWalk Robotics
- SuitX
- Ekso Bionics
- CYBERDYNE Inc.
- American Academy of Orthopaedic Surgeons
- Ottobock
- SUITX