Robotic Exoskeleton Orthotics in 2025: How Next-Gen Wearable Robotics Are Transforming Rehabilitation, Mobility, and Industry. Explore the Breakthroughs, Market Surge, and Future Outlook Shaping This High-Impact Sector.
- Executive Summary & Key Findings
- Market Size, Growth Rate & 2025–2030 Forecasts
- Major Players & Industry Landscape (e.g., eksoBionics.com, rewalk.com, suitx.com)
- Core Technologies: Sensors, Actuators, and AI Integration
- Clinical & Industrial Applications: Rehabilitation, Mobility, and Workforce Augmentation
- Regulatory Environment & Standards (e.g., fda.gov, ieee.org)
- Recent Innovations & Patent Trends
- Investment, M&A, and Funding Activity
- Challenges: Cost, Adoption Barriers, and Reimbursement
- Future Outlook: Opportunities, Risks, and Strategic Recommendations
- Sources & References
Executive Summary & Key Findings
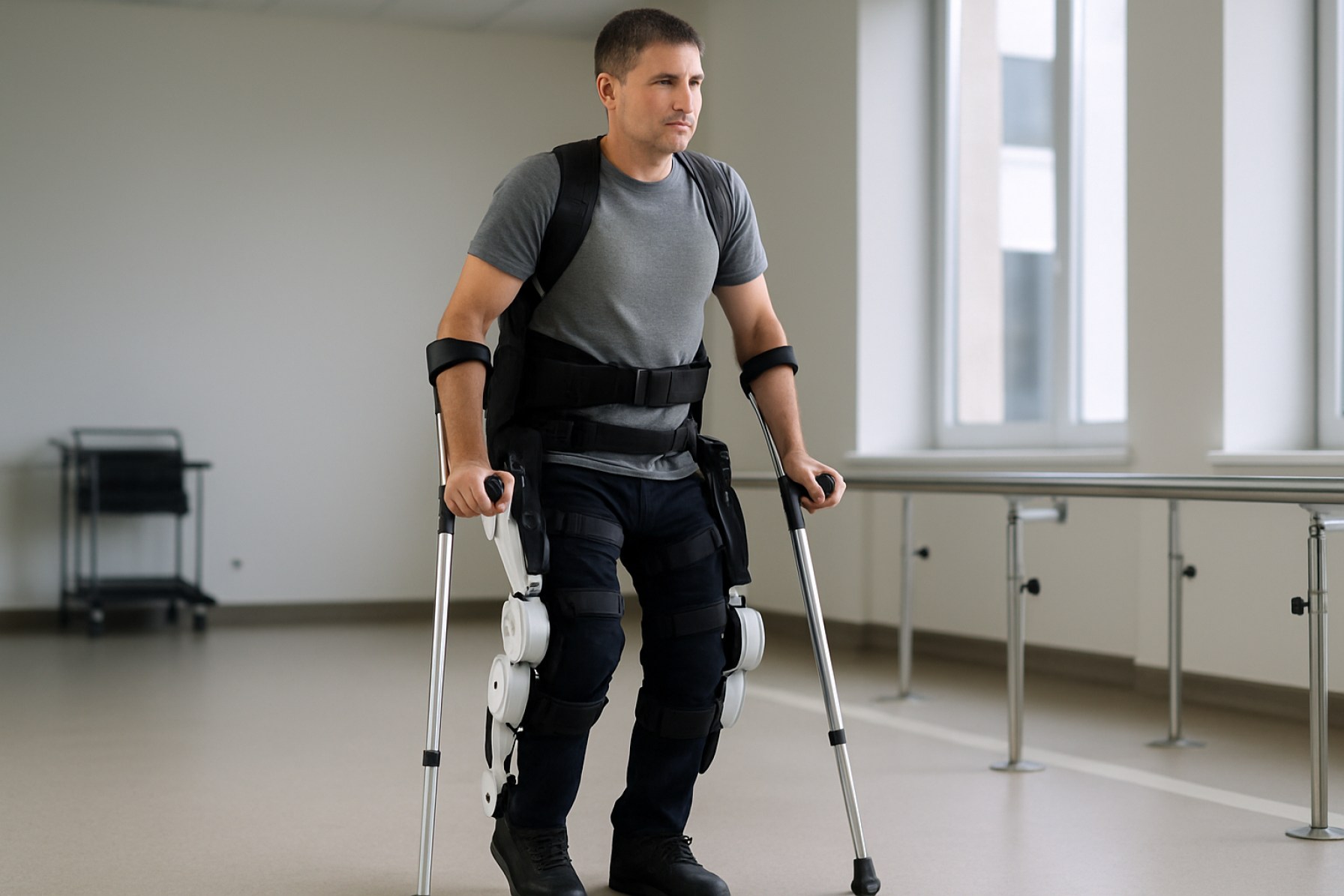
Robotic exoskeleton orthotics are rapidly transforming the landscape of rehabilitation, mobility assistance, and industrial ergonomics. As of 2025, the sector is characterized by accelerated commercialization, regulatory milestones, and expanding clinical adoption. These wearable robotic devices, designed to augment or restore human movement, are increasingly being integrated into healthcare settings for neurorehabilitation, as well as in industrial environments to reduce workplace injuries and enhance productivity.
Key industry leaders such as Ekso Bionics, ReWalk Robotics, and CYBERDYNE Inc. have continued to advance their product portfolios, focusing on both lower and upper limb exoskeletons. Ekso Bionics has reported increased adoption of its EksoNR exoskeleton in rehabilitation centers across North America and Europe, with clinical studies supporting improved outcomes for patients with spinal cord injuries and stroke. ReWalk Robotics has expanded its ReStore and ReWalk Personal systems, targeting both clinical and home use, and has received regulatory clearances in the US and EU for new device generations.
In Asia, CYBERDYNE Inc. continues to deploy its HAL (Hybrid Assistive Limb) exoskeleton in hospitals and care facilities, with ongoing research collaborations to validate long-term benefits for patients with neuromuscular disorders. Meanwhile, SuitX (now part of Ottobock) and Ottobock are expanding their industrial exoskeleton offerings, focusing on reducing musculoskeletal strain for workers in manufacturing, logistics, and construction.
Recent years have seen significant regulatory progress. The US Food and Drug Administration (FDA) and European CE marking authorities have granted clearances for several exoskeleton models, facilitating broader clinical and commercial deployment. Insurance reimbursement pathways are also evolving, with some national health systems and private insurers beginning to cover exoskeleton-assisted therapy for specific indications.
Looking ahead to the next few years, the outlook for robotic exoskeleton orthotics is highly positive. Ongoing advances in lightweight materials, battery technology, and artificial intelligence are expected to enhance device usability and patient outcomes. Industry collaborations with major healthcare providers and industrial firms are likely to accelerate, while emerging players in China and Europe are poised to introduce new competitive offerings. The sector is anticipated to see double-digit annual growth, driven by aging populations, rising rates of neurological injury, and increasing emphasis on workplace safety and productivity.
Market Size, Growth Rate & 2025–2030 Forecasts
The global market for robotic exoskeleton orthotics is poised for significant expansion in 2025 and the subsequent years, driven by technological advancements, increased clinical adoption, and growing demand in both medical and industrial sectors. As of 2025, the market is characterized by a robust pipeline of new product launches, regulatory approvals, and strategic partnerships among leading manufacturers.
Key players such as ReWalk Robotics, Ekso Bionics, CYBERDYNE Inc., and SuitX (now part of Ottobock) are at the forefront of innovation, offering exoskeletons for rehabilitation, mobility assistance, and industrial support. ReWalk Robotics continues to expand its presence in North America and Europe, with its FDA-cleared exoskeletons for spinal cord injury patients. Ekso Bionics has broadened its portfolio to include both medical and industrial exoskeletons, targeting rehabilitation centers and manufacturing environments.
In 2025, the market size is estimated to be in the low single-digit billions (USD), with projections indicating a compound annual growth rate (CAGR) exceeding 20% through 2030. This growth is fueled by increasing investments in healthcare robotics, favorable reimbursement policies in select regions, and the rising prevalence of neurological and musculoskeletal disorders. The industrial segment is also gaining momentum, as companies seek ergonomic solutions to reduce workplace injuries and enhance productivity.
Regulatory milestones are shaping the competitive landscape. For example, CYBERDYNE Inc. has achieved CE marking for its HAL exoskeleton in Europe and continues to expand its clinical applications. Ottobock, following its acquisition of SuitX, is leveraging its global distribution network to accelerate adoption in both clinical and industrial markets.
Looking ahead to 2030, the market outlook remains highly positive. Ongoing research into lightweight materials, artificial intelligence integration, and improved battery technologies is expected to further enhance device performance and user experience. As exoskeletons become more affordable and accessible, adoption rates are projected to rise across hospitals, rehabilitation centers, and industrial workplaces worldwide. The next five years will likely see increased collaboration between manufacturers, healthcare providers, and regulatory bodies to standardize protocols and expand insurance coverage, further driving market growth.
Major Players & Industry Landscape (e.g., eksoBionics.com, rewalk.com, suitx.com)
The robotic exoskeleton orthotics sector is rapidly evolving, with several major players shaping the industry landscape as of 2025. These companies are driving innovation in wearable robotics for rehabilitation, mobility assistance, and industrial applications. The competitive environment is characterized by ongoing product development, regulatory milestones, and strategic partnerships.
One of the most prominent companies is Ekso Bionics, headquartered in California. Ekso Bionics is recognized for its exoskeletons designed for both medical and industrial use. Its flagship product, the EksoNR, is FDA-cleared for use in rehabilitation of patients with stroke and spinal cord injury. The company has expanded its global footprint through collaborations with rehabilitation centers and hospitals, and continues to invest in R&D to enhance device functionality and user experience.
Another key player is ReWalk Robotics, an Israel- and US-based company specializing in wearable robotic exoskeletons for individuals with lower limb disabilities. The ReWalk Personal 6.0 system is FDA-cleared and CE-marked, enabling users with spinal cord injuries to stand and walk independently. In recent years, ReWalk has broadened its portfolio to include the ReStore soft exo-suit for stroke rehabilitation, reflecting a trend toward lighter, more versatile devices.
California-based SuitX (a subsidiary of Ottobock since 2021) is also a significant contributor to the field. SuitX focuses on modular exoskeletons for both medical and industrial applications, such as the Phoenix Medical Exoskeleton and the MAX industrial exoskeleton suite. The integration with Ottobock, a global leader in prosthetics and orthotics, has accelerated SuitX’s access to international markets and expanded its R&D capabilities.
Other notable companies include CYBERDYNE Inc. of Japan, known for its HAL (Hybrid Assistive Limb) exoskeletons, and Parker Hannifin, which developed the Indego exoskeleton for clinical and personal use. Both companies have achieved regulatory approvals in multiple regions and are actively involved in clinical research and commercialization efforts.
The industry is witnessing increased collaboration between exoskeleton manufacturers, healthcare providers, and research institutions. Regulatory progress, such as expanded FDA clearances and reimbursement pathways, is expected to further drive adoption. Looking ahead, the next few years will likely see continued innovation in device miniaturization, AI-driven control systems, and integration with digital health platforms, positioning robotic exoskeleton orthotics as a transformative force in rehabilitation and mobility solutions.
Core Technologies: Sensors, Actuators, and AI Integration
Robotic exoskeleton orthotics are rapidly advancing, driven by innovations in core technologies such as sensors, actuators, and artificial intelligence (AI) integration. As of 2025, these components are central to improving device performance, user adaptability, and clinical outcomes.
Sensor technology is foundational for exoskeletons, enabling real-time monitoring of user movement, force, and intent. Modern exoskeletons employ a combination of inertial measurement units (IMUs), force sensors, and electromyography (EMG) sensors to capture biomechanical data. For example, Ottobock integrates multi-modal sensors in its exoskeletons to enhance motion detection and safety. Similarly, CYBERDYNE Inc. utilizes bioelectrical signal sensors in its HAL (Hybrid Assistive Limb) systems, allowing the device to interpret the wearer’s voluntary muscle signals for more intuitive control.
Actuator technology has also seen significant progress. The latest exoskeletons use lightweight, high-torque electric motors and advanced transmission systems to deliver precise, responsive assistance. ReWalk Robotics employs custom-designed actuators in its wearable exoskeletons, enabling users with lower limb disabilities to stand and walk with improved stability. Pneumatic and hydraulic actuators are also being explored for applications requiring higher power output, though electric actuators remain dominant due to their efficiency and compactness.
AI integration is transforming exoskeleton orthotics by enabling adaptive, personalized assistance. Machine learning algorithms process sensor data to predict user intent, adjust support levels, and optimize gait patterns in real time. SuitX (now part of Ottobock) has developed AI-driven control systems that learn from user behavior, enhancing rehabilitation outcomes and reducing cognitive load. Ekso Bionics incorporates AI-based software in its EksoNR device, which dynamically adapts to patient progress during neurorehabilitation.
Looking ahead, the next few years are expected to bring further miniaturization of sensors, increased actuator efficiency, and more sophisticated AI algorithms. Industry leaders are investing in cloud connectivity and remote monitoring, enabling data-driven therapy and predictive maintenance. As these core technologies mature, robotic exoskeleton orthotics are poised to become more accessible, user-friendly, and effective for both clinical and industrial applications.
Clinical & Industrial Applications: Rehabilitation, Mobility, and Workforce Augmentation
Robotic exoskeleton orthotics are rapidly advancing in both clinical and industrial domains, with 2025 poised to see significant expansion in their applications for rehabilitation, mobility assistance, and workforce augmentation. These wearable devices, which integrate sensors, actuators, and advanced control systems, are designed to support or enhance human movement, offering transformative potential for individuals with mobility impairments and for workers in physically demanding environments.
In clinical rehabilitation, exoskeletons are increasingly used to assist patients recovering from spinal cord injuries, strokes, and other neurological conditions. Devices such as the Ekso Bionics EksoNR and ReWalk Robotics ReWalk Personal 6.0 are FDA-cleared for use in rehabilitation centers and, in some cases, home settings. These systems enable repetitive, task-specific gait training, which has been shown to improve walking ability and neuroplasticity in patients. In 2025, ongoing clinical trials and real-world deployments are expected to yield more robust data on long-term outcomes, with a focus on expanding indications and improving device accessibility.
Mobility assistance for individuals with lower limb paralysis or weakness is another area of rapid growth. Companies like CYBERDYNE Inc. (developer of the HAL exoskeleton) and SUITX (now part of Ottobock) are advancing lightweight, user-friendly exoskeletons that can be used in daily life. These devices are being refined for greater autonomy, improved battery life, and enhanced user comfort, with 2025 expected to see broader insurance coverage and regulatory approvals in multiple regions.
In industrial settings, exoskeletons are being adopted to reduce workplace injuries and enhance productivity. Sarcos Technology and Robotics Corporation and Ottobock are among the leaders developing powered and passive exoskeletons for logistics, manufacturing, and construction. These systems support the back, shoulders, and arms, reducing fatigue and the risk of musculoskeletal disorders. Pilot programs with major automotive and aerospace manufacturers are expanding, and 2025 is likely to see larger-scale deployments as return-on-investment data becomes available.
Looking ahead, the convergence of artificial intelligence, improved materials, and miniaturized power sources is expected to drive further innovation. The next few years will likely bring more personalized, adaptive exoskeletons, with seamless integration into clinical care pathways and industrial workflows. As costs decrease and evidence of efficacy grows, robotic exoskeleton orthotics are set to become a standard tool for enhancing human mobility and capability across diverse sectors.
Regulatory Environment & Standards (e.g., fda.gov, ieee.org)
The regulatory environment for robotic exoskeleton orthotics is rapidly evolving as these devices transition from research and pilot programs to broader clinical and commercial use. In the United States, the U.S. Food and Drug Administration (FDA) classifies most lower-limb exoskeletons as Class II medical devices, requiring premarket notification (510(k)) clearance. Notably, several exoskeletons from companies such as Ekso Bionics and ReWalk Robotics have received FDA clearance for use in rehabilitation settings and, in some cases, for personal use by individuals with spinal cord injuries. The FDA continues to update its guidance as new device types and use cases emerge, with a focus on safety, reliability, and post-market surveillance.
In Europe, robotic exoskeletons are regulated under the Medical Device Regulation (MDR 2017/745), which came fully into effect in 2021. This framework requires manufacturers to demonstrate clinical safety and performance, with conformity assessments conducted by notified bodies. Companies such as CYBERDYNE Inc. and Ottobock have obtained CE marking for their exoskeleton products, enabling distribution across the European Economic Area. The MDR places increased emphasis on post-market clinical follow-up and vigilance, which is expected to shape product development and monitoring practices through 2025 and beyond.
Internationally, standards development is being led by organizations such as the IEEE and the International Organization for Standardization (ISO). The IEEE has published standards like IEEE 11073 for health informatics and is actively working on guidelines specific to wearable robotics, including safety, interoperability, and data security. ISO/TC 299 is developing standards for robotics, including ISO 13482, which addresses safety requirements for personal care robots, a category that includes exoskeletons.
Looking ahead to 2025 and the following years, regulatory agencies are expected to refine their approaches as exoskeletons become more sophisticated and are adopted in new contexts, such as industrial workplaces and home care. The FDA has signaled interest in adaptive regulatory pathways for digital health and AI-enabled devices, which may impact exoskeletons with advanced sensing and control algorithms. Meanwhile, harmonization of standards across regions is anticipated to facilitate global market access and streamline compliance for manufacturers. Ongoing collaboration between regulators, industry leaders, and standards bodies will be crucial to ensuring safety, efficacy, and innovation in the rapidly advancing field of robotic exoskeleton orthotics.
Recent Innovations & Patent Trends
The field of robotic exoskeleton orthotics has experienced significant innovation in recent years, with 2025 marking a period of rapid technological advancement and increased patent activity. These wearable robotic devices, designed to augment or restore human mobility, are being refined for both medical rehabilitation and industrial applications. The convergence of lightweight materials, advanced sensors, and AI-driven control systems is driving a new generation of exoskeletons that are more adaptive, user-friendly, and effective.
Leading manufacturers such as Ekso Bionics and ReWalk Robotics have introduced next-generation exoskeletons with enhanced ergonomics and improved gait assistance algorithms. In 2024 and 2025, Ekso Bionics launched updated versions of its EksoNR and Ekso Indego systems, incorporating real-time feedback and cloud-based data analytics to personalize therapy and track patient progress. Similarly, ReWalk Robotics has expanded its product line to include soft exosuits, which use textile-based actuators for lighter, more flexible support, particularly for stroke and spinal cord injury rehabilitation.
Patent filings in this sector have surged, reflecting both the competitive landscape and the pace of innovation. According to data from the United States Patent and Trademark Office, the number of patents granted for exoskeleton technologies has doubled over the past five years, with a notable increase in filings related to AI-powered control systems, modular designs, and energy-efficient actuation. Companies such as CYBERDYNE Inc. and Hocoma AG are actively patenting novel sensor integration methods and adaptive control algorithms, aiming to improve user safety and device responsiveness.
Industrial exoskeletons are also seeing rapid development, with firms like Ottobock and SuitX (now part of Ottobock) focusing on reducing workplace injuries and enhancing worker endurance. Their recent patents emphasize lightweight frame construction and intuitive user interfaces, making exoskeletons more practical for daily use in logistics, manufacturing, and construction.
Looking ahead, the outlook for robotic exoskeleton orthotics is robust. The integration of machine learning for adaptive assistance, wireless connectivity for remote monitoring, and modularity for customizable support are expected to dominate patent activity through 2025 and beyond. As regulatory pathways become clearer and clinical evidence mounts, broader adoption in both healthcare and industry is anticipated, positioning exoskeletons as a transformative technology in mobility and rehabilitation.
Investment, M&A, and Funding Activity
The robotic exoskeleton orthotics sector is experiencing robust investment and deal activity as the technology matures and clinical adoption accelerates. In 2025, venture capital, strategic corporate investments, and public funding continue to drive innovation and commercialization, with a focus on both medical and industrial applications.
Key industry players such as Ekso Bionics, ReWalk Robotics, and CYBERDYNE Inc. have attracted significant funding rounds in recent years, supporting product development and global market expansion. Ekso Bionics, a pioneer in wearable exoskeletons for rehabilitation and industrial use, has leveraged both private placements and public offerings to raise capital for scaling manufacturing and R&D. ReWalk Robotics, known for its FDA-cleared exoskeletons for spinal cord injury patients, has also secured additional funding to expand its product portfolio and enter new markets.
Mergers and acquisitions are shaping the competitive landscape. Strategic acquisitions by larger medical device companies and technology conglomerates are expected to increase through 2025, as established firms seek to integrate exoskeleton solutions into broader rehabilitation and mobility portfolios. For example, CYBERDYNE Inc. has expanded its reach through partnerships and investments in rehabilitation centers, while also exploring joint ventures in Asia and Europe.
Government and institutional funding remain crucial, particularly in Europe and Asia, where public health systems are investing in robotic rehabilitation technologies to address aging populations and workforce shortages. In the United States, the Department of Veterans Affairs and other agencies continue to support clinical trials and pilot programs for exoskeleton orthotics, further validating the sector and attracting private capital.
Looking ahead, the outlook for investment and M&A activity in robotic exoskeleton orthotics is positive. The sector is expected to see continued inflows from both traditional medtech investors and new entrants from the robotics and AI fields. As clinical evidence grows and reimbursement pathways become clearer, more companies are likely to pursue IPOs or strategic exits. The next few years will likely witness increased consolidation, with leading innovators such as Ekso Bionics, ReWalk Robotics, and CYBERDYNE Inc. at the forefront of deal activity and technological advancement.
Challenges: Cost, Adoption Barriers, and Reimbursement
Robotic exoskeleton orthotics have demonstrated significant potential in rehabilitation, mobility assistance, and industrial applications. However, as of 2025, the sector faces persistent challenges related to cost, adoption barriers, and reimbursement, which collectively impact the pace and breadth of market penetration.
Cost remains a primary obstacle. Advanced exoskeletons, such as those developed by Ekso Bionics, ReWalk Robotics, and CYBERDYNE Inc., often retail for tens of thousands of dollars per unit. The high price reflects the complexity of hardware, integration of sensors and actuators, and the need for robust safety features. For example, the EksoNR and ReWalk Personal 6.0 systems are priced in the $70,000–$100,000 range, making them inaccessible for many individuals and smaller clinics. While some manufacturers are working to reduce costs through modular designs and economies of scale, significant price drops are not expected in the immediate future.
Adoption barriers are multifaceted. Clinicians and rehabilitation centers often require extensive training to safely and effectively operate exoskeletons, and workflow integration can be disruptive. There is also a need for more robust, long-term clinical evidence to support widespread adoption. While companies like Ottobock and Hocoma (a DIH company) have invested in education and support programs, the learning curve and perceived complexity remain significant hurdles. Additionally, device size, weight, and adaptability to different patient anatomies continue to limit use in some populations.
Reimbursement is a critical challenge, particularly in the United States and Europe. As of 2025, only a limited number of exoskeletons have received regulatory clearance for reimbursement. For instance, ReWalk Robotics achieved a milestone in 2023 when the U.S. Centers for Medicare & Medicaid Services (CMS) granted a unique Healthcare Common Procedure Coding System (HCPCS) code for its personal exoskeleton, but broad coverage remains limited and subject to case-by-case review. Insurers often require extensive documentation of medical necessity and evidence of functional improvement, which can delay or prevent access for many users. Efforts by industry groups and manufacturers to expand reimbursement are ongoing, but significant progress is expected to be incremental over the next few years.
Looking ahead, the sector is likely to see gradual improvements in affordability, usability, and insurance coverage, driven by continued advocacy, technological innovation, and accumulating clinical data. However, overcoming the intertwined challenges of cost, adoption, and reimbursement will remain central to the widespread integration of robotic exoskeleton orthotics through the remainder of the decade.
Future Outlook: Opportunities, Risks, and Strategic Recommendations
The future outlook for robotic exoskeleton orthotics in 2025 and the following years is marked by rapid technological advancements, expanding clinical applications, and evolving regulatory landscapes. As the global population ages and the prevalence of mobility-impairing conditions rises, demand for advanced orthotic solutions is expected to surge. Robotic exoskeletons, which augment or restore mobility for individuals with neurological or musculoskeletal impairments, are positioned at the forefront of this transformation.
Key industry players such as Ekso Bionics, ReWalk Robotics, and CYBERDYNE Inc. are intensifying research and development efforts to enhance device functionality, user comfort, and affordability. For instance, Ekso Bionics continues to expand its clinical and industrial exoskeleton offerings, focusing on improved ergonomics and adaptive control systems. ReWalk Robotics is advancing wearable exoskeletons for spinal cord injury rehabilitation, with ongoing clinical trials and collaborations with rehabilitation centers worldwide. Meanwhile, CYBERDYNE Inc. is leveraging its Hybrid Assistive Limb (HAL) technology to support both medical and industrial applications, with regulatory approvals in several markets.
Opportunities in the sector are driven by increasing integration of artificial intelligence and machine learning, which enable more intuitive and responsive exoskeleton control. The adoption of lightweight materials and modular designs is expected to further improve user experience and broaden the range of treatable conditions. Additionally, partnerships between manufacturers and healthcare providers are facilitating the development of tailored rehabilitation protocols and expanding access to exoskeleton-assisted therapy.
However, several risks persist. High device costs and limited reimbursement frameworks remain significant barriers to widespread adoption, particularly in emerging markets. Regulatory challenges, including the need for robust clinical evidence and compliance with evolving safety standards, may delay product launches and market entry. Furthermore, ensuring long-term device reliability and user safety is critical, especially as exoskeletons transition from controlled clinical environments to everyday use.
Strategic recommendations for stakeholders include prioritizing collaborative research to generate clinical data supporting efficacy and safety, engaging with regulatory bodies to streamline approval processes, and investing in scalable manufacturing to reduce costs. Companies should also focus on user-centric design and post-market surveillance to address real-world challenges and foster trust among clinicians and patients. As the sector matures, ongoing innovation and cross-sector partnerships will be essential to unlock the full potential of robotic exoskeleton orthotics and deliver meaningful improvements in mobility and quality of life.
Sources & References
- ReWalk Robotics
- CYBERDYNE Inc.
- SuitX
- Ottobock
- Ekso Bionics
- Ekso Bionics
- ReWalk Robotics
- SuitX
- Sarcos Technology and Robotics Corporation
- IEEE
- Hocoma AG