Table of Contents
- Executive Summary: 2025 Market Snapshot & Key Takeaways
- Current State of Wearable Exoskeleton Rehabilitation Devices: Leading Players & Innovations
- Market Size & Forecast (2025–2030): Growth Projections and Regional Analysis
- Emerging Technologies: Advances in Sensors, Materials, and AI Integration
- Clinical Applications: From Stroke Recovery to Spinal Cord Injury Rehabilitation
- Regulatory Landscape & Standards: Navigating Global Approvals & Compliance
- Investment & Funding Trends: Startups, M&A, and Strategic Partnerships
- End-User Perspectives: Hospitals, Clinics, and Home-Based Rehabilitation
- Key Challenges: Affordability, Accessibility, and User Adoption Barriers
- Future Outlook: Game-Changing Developments and Industry Roadmap to 2030
- Sources & References
Executive Summary: 2025 Market Snapshot & Key Takeaways
The wearable exoskeleton rehabilitation device market in 2025 is experiencing robust growth, fueled by technological innovation, expanding clinical evidence, and increasing demand for advanced mobility solutions for individuals with neurological and musculoskeletal impairments. As of 2025, leading manufacturers continue to scale operations and deploy devices across rehabilitation centers, hospitals, and increasingly into outpatient and home environments.
Key industry players such as Ekso Bionics, ReWalk Robotics, and CYBERDYNE Inc. are at the forefront, leveraging artificial intelligence, improved sensor technology, and lighter materials to enhance device performance and patient outcomes. These companies report a notable rise in adoption rates, with exoskeleton-assisted therapy sessions becoming a standard adjunct in neurorehabilitation for conditions such as stroke, spinal cord injury, and multiple sclerosis.
Recent events in 2025 include the launch of next-generation models featuring adaptive gait algorithms and remote monitoring capabilities, furthering the shift towards patient-centric, data-driven rehabilitation. Ekso Bionics continues to expand its clinical footprint, securing new partnerships with rehabilitation networks in North America and Europe. Similarly, ReWalk Robotics has intensified efforts to secure reimbursement and regulatory approvals, facilitating broader access for home use. Meanwhile, CYBERDYNE Inc. is advancing its HAL (Hybrid Assistive Limb) technology, emphasizing real-time biofeedback integration.
Market data from industry bodies indicates mid-to-high double-digit growth rates in shipment volumes and clinical installations for 2025, reflecting increased confidence among healthcare providers in the clinical efficacy and cost-benefit profile of wearable exoskeletons. The sector is also witnessing early signs of adoption in emerging markets in Asia-Pacific and the Middle East, driven by demographic trends and government-led rehabilitation initiatives.
Looking ahead to the next few years, the market outlook remains positive. Continued investment in R&D is expected to yield devices with enhanced battery life, greater modularity, and improved affordability. Strategic collaborations between device manufacturers and healthcare systems are likely to accelerate, supporting expanded clinical trials and real-world evidence generation. As regulatory frameworks mature and reimbursement pathways become more defined, the wearable exoskeleton rehabilitation device sector is poised for broader adoption, with a growing emphasis on patient quality of life and long-term outcomes.
Current State of Wearable Exoskeleton Rehabilitation Devices: Leading Players & Innovations
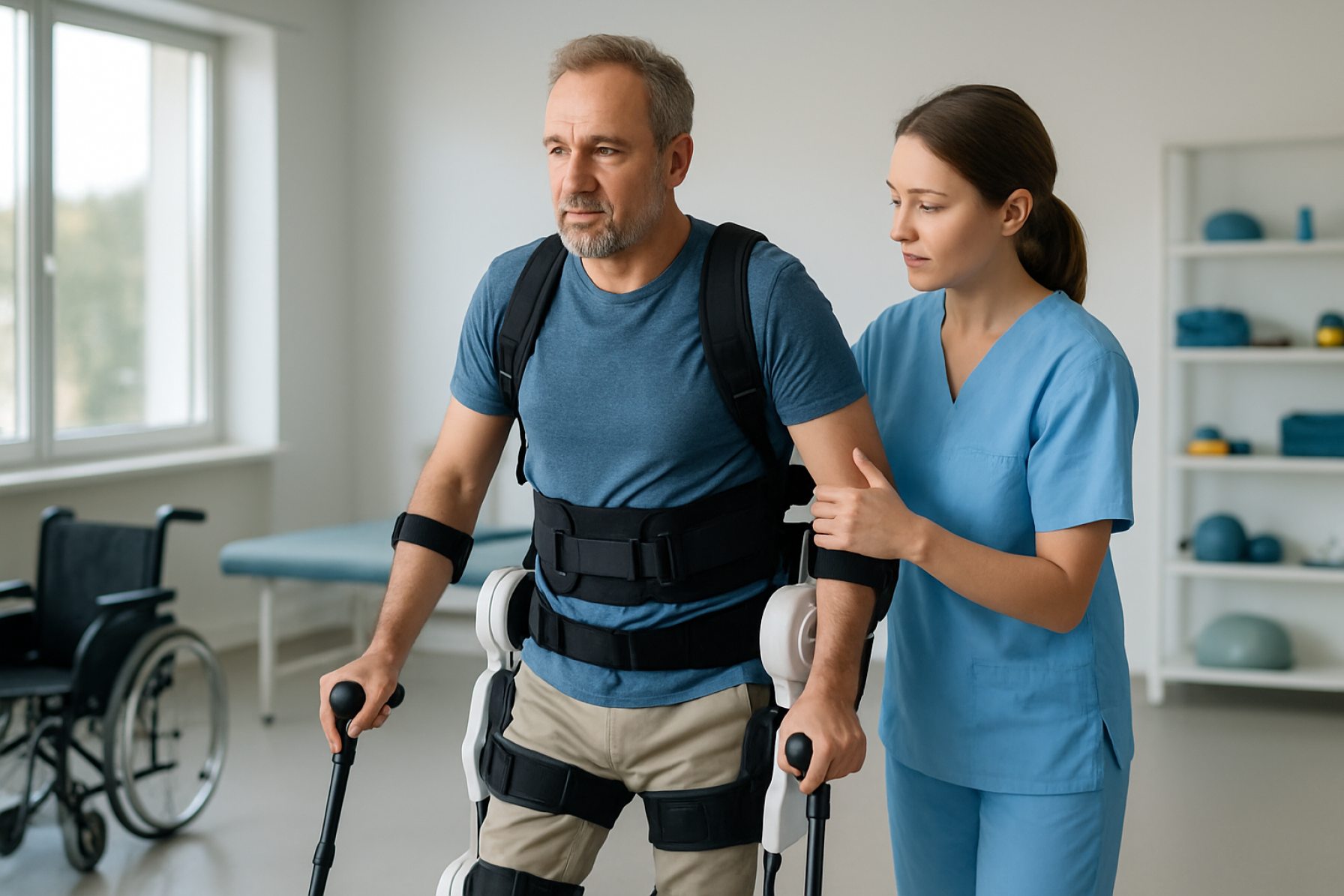
The wearable exoskeleton rehabilitation devices sector has evolved rapidly, with 2025 marking a period of intensified activity among both established manufacturers and emerging innovators. These powered or passive exoskeletons, designed to assist or restore mobility in patients with neurological or musculoskeletal impairments, are now increasingly found in rehabilitation centers, hospitals, and even home settings.
Leading the global market, Ekso Bionics continues to deploy its EksoNR exoskeleton, which is FDA-cleared for use with patients suffering from stroke, spinal cord injury, and acquired brain injury. Their devices are being integrated into clinical protocols at major rehabilitation networks, reflecting growing acceptance and clinical validation. Similarly, ReWalk Robotics has expanded its portfolio for both clinical and personal use, notably with its ReStore soft exosuit for post-stroke gait training. The company’s focus on insurance coverage and regulatory approvals is increasing accessibility in North America and Europe.
In Asia, CYBERDYNE Inc. remains a front-runner, with its Hybrid Assistive Limb (HAL) exoskeleton deployed in rehabilitation centers and hospitals across Japan and parts of Europe. The company’s ongoing research partnerships are paving the way for more adaptive, AI-driven exoskeletons tailored to individual patient needs.
Other notable players include Hocoma, whose Lokomat system—though primarily a robotic gait trainer—bridges the gap between exoskeletons and advanced rehabilitation robotics. Parker Hannifin Corporation further advances the sector with its Indego exoskeleton, emphasizing modular design and lightweight components, suitable for both clinical and at-home rehabilitation.
Recent innovations focus on improved ergonomics, lighter materials, and the integration of machine learning algorithms to personalize therapy. Companies are increasingly collaborating with healthcare providers to gather real-world data, refine device usability, and demonstrate long-term functional outcomes. Notably, the trend toward wearable, soft-exosuit technology—such as that pursued by ReWalk Robotics—is expected to increase patient comfort and expand eligible user groups.
Looking ahead to the next few years, the sector is anticipated to witness broader adoption due to ongoing clinical trials, further reductions in device cost, and greater insurance reimbursement. The convergence of sensors, AI, and cloud-based analytics promises to enable real-time progress tracking and adaptive therapy protocols, potentially revolutionizing post-acute and long-term rehabilitation landscapes.
Market Size & Forecast (2025–2030): Growth Projections and Regional Analysis
The global market for wearable exoskeleton rehabilitation devices is anticipated to experience robust growth from 2025 through 2030, propelled by ongoing technological advancements, an aging population, and increasing prevalence of neurological and musculoskeletal disorders. The sector’s expansion is further enabled by rising investments, supportive regulatory environments, and the progressive integration of exoskeletons into clinical and home rehabilitation settings.
In 2025, leading industry participants such as Ekso Bionics, ReWalk Robotics, and Cyberdyne are expected to maintain their positions at the forefront, supported by ongoing product launches and increased clinical validation. The market value is projected to escalate, with estimates placing the sector’s worth in the mid single-digit billion USD range by the end of the forecast period. Growth rates are expected to show a double-digit compound annual growth rate (CAGR), with some regional variations driven by adoption rates and reimbursement policies.
Geographically, North America is anticipated to retain its lead in market share through 2030, underpinned by strong healthcare infrastructure, favorable reimbursement trends, and the presence of pioneering manufacturers such as Ekso Bionics and ReWalk Robotics. Meanwhile, Europe will continue to be a significant market, benefiting from initiatives to integrate robotic rehabilitation into standard care pathways and the activities of companies like Ottobock. Asia-Pacific is forecasted as the fastest-growing region, with Japan’s Cyberdyne and China’s emerging manufacturers accelerating adoption due to increasing patient awareness and government healthcare investments.
Key market drivers in 2025 and beyond include the expansion of exoskeleton indications beyond spinal cord injury to encompass stroke, multiple sclerosis, and elderly mobility support. The transition from hospital-based to outpatient and home rehabilitation models is expected to boost device uptake. Additionally, partnerships between exoskeleton developers and healthcare providers are enhancing device accessibility and affordability. For example, Ekso Bionics and ReWalk Robotics have both announced collaborations with rehabilitation centers to deploy devices in wider clinical settings.
Looking ahead to 2030, continued advancements in lightweight materials, artificial intelligence integration, and cost reductions are likely to drive broader adoption. As regulatory agencies refine approval pathways and reimbursement expands, wearable exoskeleton rehabilitation devices are poised for sustained, dynamic growth across diverse global markets.
Emerging Technologies: Advances in Sensors, Materials, and AI Integration
Wearable exoskeleton rehabilitation devices are poised for significant transformation through advances in sensor technology, materials science, and artificial intelligence (AI) integration. These innovations are converging to deliver more adaptive, effective, and user-friendly solutions for patients with mobility impairments, particularly in the realm of neurorehabilitation and post-injury recovery.
In 2025, the sector is witnessing rapid adoption of high-precision sensor arrays—including inertial measurement units (IMUs), electromyography (EMG) sensors, and force sensors—that enable real-time monitoring of user movements and physiological signals. Such technologies are foundational for personalized therapy and safety. Leading manufacturers such as Ekso Bionics and ReWalk Robotics have incorporated multi-modal sensor suites in their latest exoskeletons, facilitating enhanced feedback loops and adaptive assistance tailored to individual patient needs.
Material science breakthroughs are further shaping the landscape. The adoption of lightweight, durable composites and soft robotics elements is enabling exoskeletons to become less bulky and more comfortable for prolonged use. Companies like Hocoma are integrating flexible actuators and breathable, ergonomic fabrics to increase patient compliance and expand applicability beyond clinical settings into home and community environments.
AI-driven control systems are arguably the most transformative development currently underway. Machine learning algorithms now process data streams from embedded sensors to predict user intentions, dynamically adjust assistance levels, and optimize gait patterns. For instance, SuitX and CYBERDYNE are advancing AI-enabled exoskeletons that adapt in real-time to changes in user performance and rehabilitation progress. This shift from static pre-set programs toward intelligent, context-aware assistance is expected to improve therapeutic outcomes significantly.
Looking toward the next several years, these trends are set to accelerate. Industry bodies anticipate broader integration of cloud connectivity and remote monitoring, allowing clinicians to track patient progress and fine-tune device parameters from afar. Additionally, ongoing R&D efforts are focusing on the seamless combination of neural interfaces with wearable exoskeletons, aiming to close the loop between brain signals and mechanical assistance. The convergence of smart sensors, advanced materials, and AI is expected to drive the next wave of market growth and clinical adoption, making wearable exoskeleton rehabilitation devices a cornerstone of modern neurorehabilitation and mobility restoration.
Clinical Applications: From Stroke Recovery to Spinal Cord Injury Rehabilitation
Wearable exoskeleton rehabilitation devices have seen rapid clinical adoption and technological refinement, particularly in the treatment and management of neuromuscular conditions such as stroke and spinal cord injury (SCI). As of 2025, these robotic assistive systems are increasingly integrated into rehabilitation protocols worldwide, driven by mounting clinical evidence and expanding accessibility. Leading manufacturers have developed exoskeletons that support both upper and lower limb mobility, targeting diverse patient populations across settings including hospitals, outpatient clinics, and even home environments.
In stroke recovery, wearable exoskeletons facilitate intensive, repetitive gait and movement training—a critical factor in neuroplasticity and functional improvement. Clinical trials and real-world deployments have demonstrated that devices such as the ReWalk Personal 6.0 and EksoNR enable earlier mobilization, greater walking distances, and improved independence compared to conventional therapy alone. These systems offer adjustable levels of assistance, allowing therapists to tailor sessions to individual patient abilities and progress, which has been shown to enhance engagement and outcomes.
For individuals with spinal cord injury, exoskeleton-assisted walking has become a transformative intervention. Patients with motor-complete and incomplete injuries are now utilizing devices like those from ReWalk Robotics, Ekso Bionics, and CYBERDYNE to participate in standing and walking activities during rehabilitation. Such interventions are correlated with secondary health benefits, including improved cardiovascular function, reduced spasticity, better bowel and bladder management, and psychological well-being. In 2025, new-generation exoskeletons emphasize intuitive control, lighter materials, and enhanced safety features, making them suitable for broader deployment and longer daily use.
The clinical utility of exoskeletons is expanding beyond traditional neurological indications. Therapists are increasingly employing these devices for rehabilitation in orthopedic injury, multiple sclerosis, and even in geriatric populations to prevent falls and maintain mobility. Wearable exoskeletons from companies like SuitX and BIONIK Laboratories are being tested in multi-center trials and are expected to receive further regulatory clearances in the coming years.
Looking ahead, the next few years will likely see further integration of exoskeletons with digital health platforms, real-time gait analytics, and cloud-based patient tracking. Ongoing partnerships between device manufacturers and major rehabilitation networks are expected to generate robust longitudinal data, informing best practices and optimizing device algorithms. As costs gradually decrease and insurance coverage expands, wearable exoskeletons are poised to become a mainstream component of neurorehabilitation, offering new hope for functional recovery and independence to millions worldwide.
Regulatory Landscape & Standards: Navigating Global Approvals & Compliance
The regulatory landscape for wearable exoskeleton rehabilitation devices is undergoing significant evolution in 2025, as global demand for these technologies accelerates across medical and industrial applications. These devices, which assist patients with mobility impairments or facilitate rehabilitation after injury, are subject to stringent oversight to ensure safety, efficacy, and interoperability. Regulatory requirements vary by region, with principal markets including the United States, European Union, and Asia-Pacific countries implementing or updating standards to address the unique challenges posed by exoskeleton technologies.
In the United States, wearable exoskeletons intended for medical use are typically classified as Class II medical devices, requiring premarket notification through the FDA’s 510(k) process. In recent years, the FDA has granted clearances to several exoskeleton systems, such as those developed by Ekso Bionics, ReWalk Robotics, and Cyberdyne. As the number and complexity of exoskeletons increase, the FDA is actively engaging with manufacturers to clarify expectations around clinical evidence, risk management, and post-market surveillance.
The European Union, under the Medical Device Regulation (MDR) framework, has also refined its approach to exoskeleton certification. Manufacturers must demonstrate compliance with essential requirements related to safety, performance, and cybersecurity, often involving Notified Body assessment and clinical evaluations. Several companies, including Ottobock and Hocoma, have obtained CE marking for their rehabilitation exoskeletons, paving the way for broader adoption in European healthcare settings.
In Asia-Pacific, regulatory approval processes are diverse but converging towards harmonized standards. In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) has approved the HAL system from Cyberdyne, while China is expanding its regulatory pathways in line with international best practices. The growing presence of regional manufacturers and cross-border collaborations—such as SuitX, now part of Ottobock—highlights the importance of global compliance strategies.
Looking ahead, regulatory bodies are expected to introduce updated guidelines and standards addressing software validation, human-machine interface safety, and data privacy, reflecting the increasingly digital and connected nature of exoskeletons. The International Organization for Standardization (ISO) is working on specific technical standards for robotic exoskeletons, projected to be influential by 2026 and beyond. Industry leaders and stakeholders are collaborating with regulators to ensure that innovation can proceed alongside robust patient safety and device reliability requirements.
Investment & Funding Trends: Startups, M&A, and Strategic Partnerships
The wearable exoskeleton rehabilitation devices sector is experiencing robust investment and partnership activity in 2025, driven by rising demand for advanced neurorehabilitation solutions and an aging global population. Startups in this space continue to attract venture capital, particularly those developing lightweight, versatile, and AI-enhanced exoskeletons for both clinical and home-based rehabilitation.
Companies such as ReWalk Robotics Ltd. and Ekso Bionics Holdings, Inc. have maintained their positions as leading innovators, while also serving as magnets for strategic investments. In early 2025, Ekso Bionics Holdings, Inc. announced a fresh round of funding aimed at expanding its clinical trial programs and accelerating commercialization of its EksoNR rehabilitation exoskeleton. Similarly, ReWalk Robotics Ltd. reported new investments to support further development of its ReStore and Personal 6.0 exoskeletons, targeting both spinal cord injury and stroke rehabilitation markets.
Startups are also entering the market with novel approaches. Notably, CYBERDYNE Inc., a Japanese pioneer in medical exoskeletons, has renewed its global partnerships in 2025 to broaden the reach of its HAL (Hybrid Assistive Limb) technology. These collaborations are designed to facilitate clinical studies and pave the way for regulatory approval in new geographies.
The sector has witnessed several mergers and acquisitions (M&A) as established medical device firms seek to bolster their portfolios. In 2024 and early 2025, the acquisition of smaller exoskeleton startups by larger rehabilitation equipment manufacturers has accelerated. For example, Ottobock SE & Co. KGaA, a global leader in prosthetics and orthotics, has continued its strategy of integrating wearable robotics into its product lines through targeted investments and acquisitions.
Strategic partnerships between exoskeleton developers and rehabilitation clinics or hospital networks are another key trend. These alliances enable real-world validation, facilitate reimbursement discussions, and drive large-scale adoption. For instance, in 2025, Ekso Bionics Holdings, Inc. expanded collaborations with U.S. and European rehabilitation centers to pilot next-generation devices, focusing on usability and patient outcomes.
Outlook for the next several years suggests sustained growth, with global investment flowing towards AI-powered adaptive exoskeletons, modular designs for diverse patient needs, and integration with telemedicine platforms. As device manufacturers continue to forge strategic partnerships and pursue M&A opportunities, the wearable exoskeleton rehabilitation market is poised for technological advancement and broader clinical adoption by 2027.
End-User Perspectives: Hospitals, Clinics, and Home-Based Rehabilitation
In 2025, wearable exoskeleton rehabilitation devices are increasingly being adopted across diverse end-user settings, notably hospitals, specialized rehabilitation clinics, and the home environment. This adoption is propelled by continued advancements in robotics, miniaturization, and user-centered design, enabling exoskeletons to address a range of neurological and musculoskeletal impairments, such as those resulting from stroke, spinal cord injuries, or age-related mobility loss.
Hospitals and large rehabilitation centers remain the primary adopters of advanced exoskeleton systems, leveraging their capabilities for intensive, repetitive gait and upper limb training. Devices like the Ekso Bionics EksoNR and ReWalk Robotics ReWalk Personal 6.0 are being regularly utilized for gait rehabilitation and early mobilization within inpatient and outpatient settings, where clinical staff can monitor progress and adjust protocols. The integration of real-time data analytics and cloud connectivity is enhancing clinicians’ ability to personalize therapy regimens and objectively track recovery trajectories.
Specialized clinics are also witnessing increased integration of wearable exoskeletons tailored to specific patient populations. For instance, Cyberdyne’s HAL (Hybrid Assistive Limb) system is being deployed in neurological rehabilitation centers, offering precise assistance and biofeedback for patients with partial motor function. Clinics often favor modular and lighter-weight exoskeletons that support both upper and lower limb rehabilitation, facilitating broader patient access and maximizing device utilization.
A notable trend in 2025 is the migration of exoskeleton technology into home-based rehabilitation, enabled by recent innovations in portability, affordability, and ease of use. Companies such as Parker Hannifin (Indego Personal) and ReWalk Robotics are providing personal exoskeletons designed for independent daily use, with remote supervision by therapists via telehealth platforms. This shift allows for intensive therapy beyond the clinic, promoting patient autonomy and extending the rehabilitation continuum into daily life. Early pilot programs and case studies indicate improved adherence to therapy regimens and positive functional outcomes, although long-term, large-scale data are still being gathered.
Looking forward, end-user perspectives suggest continued demand for lighter, more intuitive, and cost-effective devices. Hospitals and clinics are seeking exoskeletons that integrate seamlessly with electronic health records and rehabilitation software, while home users prioritize safety, simplicity, and customer support. The convergence of wearable robotics, digital health tools, and remote monitoring is expected to further blur the boundaries between clinical and home-based rehabilitation over the next few years, shaping a more personalized and accessible landscape for recovery.
Key Challenges: Affordability, Accessibility, and User Adoption Barriers
Wearable exoskeleton rehabilitation devices have made significant technological strides, but widespread adoption in 2025 and the near future remains hampered by persistent challenges—primarily affordability, accessibility, and user adoption barriers. The high cost of development, manufacturing, and maintenance is a major hurdle. Leading companies such as Ekso Bionics and ReWalk Robotics offer advanced exoskeletons for medical and rehabilitation use, but device prices often range from $50,000 to $100,000 or more. This price point limits accessibility for many patients, clinics, and rehabilitation centers, especially in regions without strong insurance reimbursement or public funding mechanisms.
Insurance coverage for wearable exoskeletons remains inconsistent across markets. While select devices have achieved local approvals and coverage—such as ReWalk Robotics’s exoskeleton being eligible for reimbursement in Germany and select U.S. cases—the majority of users must rely on grants, clinical trials, or out-of-pocket payment. This creates disparities in access, particularly for patients in countries lacking structured reimbursement pathways.
Accessibility is further impeded by the need for specialized clinical settings and trained personnel to supervise exoskeleton use. Devices from companies like CYBERDYNE Inc. and Hocoma AG are primarily deployed in rehabilitation hospitals and research centers due to their complexity, size, and safety requirements. Efforts are underway to develop more lightweight, user-friendly, and home-compatible exoskeletons, but in 2025, at-home use is not yet widespread and is largely limited to pilot programs or early adopters.
User adoption also faces ergonomic and psychological barriers. Some patients experience discomfort or fatigue with prolonged use, and device fitting must be tailored to individual body types and needs. Furthermore, acceptance among clinicians and therapists is influenced by the availability of robust clinical evidence demonstrating clear patient benefits, safety, and outcomes. Companies such as Ekso Bionics and ReWalk Robotics are investing in clinical studies, but broad consensus in the medical community may take additional years to solidify.
Looking forward, industry leaders are pursuing cost-reduction strategies through modular design, increased automation in manufacturing, and scalable production. There is also a push for greater collaboration with healthcare providers and insurers to develop new payment models and evidence-based reimbursement pathways. Overcoming these key challenges will be critical to expanding the societal impact of wearable exoskeleton rehabilitation devices in the next few years.
Future Outlook: Game-Changing Developments and Industry Roadmap to 2030
The trajectory of wearable exoskeleton rehabilitation devices entering 2025 signals a pivotal era marked by rapid technological advancement, expanding clinical validation, and broader healthcare adoption. As the global population ages and neurological and musculoskeletal disorders become increasingly prevalent, the demand for robust, user-friendly, and cost-effective exoskeleton solutions is intensifying.
One of the most significant near-term developments is the anticipated integration of artificial intelligence (AI) and adaptive machine learning algorithms. These technologies are being leveraged to enable real-time customization of exoskeleton assistance, enhancing gait and movement patterns tailored to individual patient needs. Leading manufacturers such as ReWalk Robotics and Ekso Bionics are advancing AI-driven control systems that support more naturalistic mobility and improved rehabilitation outcomes.
Another key trend for 2025 and beyond is the miniaturization and modularization of exoskeleton components. Companies including Ottobock and CYBERDYNE are focused on producing lighter, less obtrusive devices that can be adapted for use in both clinical and community settings. This shift is expected to lower barriers to everyday use, helping patients integrate exoskeleton-assisted rehabilitation into their daily routines more seamlessly.
Data-driven remote monitoring and tele-rehabilitation capabilities are also gaining traction. Wearable exoskeletons equipped with wireless connectivity and advanced sensor arrays allow clinicians to track patient progress and adjust therapy protocols remotely. This feature is particularly valuable in extending access to rehabilitation services for patients in underserved or rural regions.
From a regulatory perspective, streamlined approval pathways and growing reimbursement policies are poised to accelerate adoption. Key industry players are working closely with healthcare authorities to demonstrate device safety, efficacy, and cost-effectiveness through large-scale clinical trials, with an eye toward broader insurance coverage within the next few years.
Looking ahead to 2030, many industry analysts foresee the convergence of exoskeletons with digital health ecosystems, including integration with electronic health records (EHRs) and personalized digital therapeutics. The expansion of collaborative industry consortia and public-private partnerships is expected to foster innovation, standardize protocols, and drive down costs, making wearable exoskeleton rehabilitation devices accessible to a much wider patient population worldwide.