Table of Contents
- Executive Summary: 2025 Industry Snapshot
- Technology Overview: Principles of Backscatter Tomographic Bioluminescence Imaging
- Key Players & Innovators: Leading Companies and Organizations
- Current Applications in Preclinical and Clinical Settings
- Market Size, Growth Drivers, and 2025–2030 Forecasts
- Emerging Trends: AI Integration and Next-Generation Hardware
- Regulatory Landscape and Compliance Updates
- Competitive Analysis: Opportunities and Barriers to Entry
- Future Outlook: Disruptive Potential Through 2030
- References & Official Resources
- Sources & References
Executive Summary: 2025 Industry Snapshot
Backscatter Tomographic Bioluminescence Imaging (BTBI) is emerging as a pivotal modality in the life sciences and preclinical imaging sectors, driven by a convergence of advances in photonic detection, molecular biology, and computational reconstruction. As of 2025, the industry is witnessing a marked acceleration in the adoption of BTBI technologies, particularly within translational research and drug discovery pipelines. The modality addresses key limitations of conventional bioluminescence imaging (BLI), offering greater spatial resolution and target localization through tomographic reconstruction and backscatter signal analysis.
Several leading instrument manufacturers have introduced next-generation BTBI platforms designed for high-throughput small animal imaging. Notably, PerkinElmer, Inc. and Bruker Corporation have both highlighted advanced optical tomography modules in their recent system upgrades, integrating sensitive CCD/CMOS detectors and proprietary reconstruction algorithms to deliver three-dimensional bioluminescence mapping. These systems are enabling researchers to visualize deeper tissue signals with improved quantification, a capability critical for oncology, infectious disease, and gene therapy studies.
A significant trend in 2025 is the integration of BTBI with multi-modal imaging suites. Companies are increasingly offering hybrid systems that combine bioluminescence tomography with fluorescence, X-ray, or MRI modalities, facilitating comprehensive anatomical and functional data collection from a single imaging session. Miltenyi Biotec, for example, has expanded its imaging portfolio to support synchronized acquisition and co-registration of multiple imaging contrasts, reflecting growing demand from academic and pharmaceutical research centers for holistic in vivo analysis.
On the data analytics front, AI-powered image reconstruction and automated region-of-interest quantification have become standard features in BTBI platforms, reducing analysis time and enhancing reproducibility. Strategic collaborations between equipment vendors and cloud-based analytics providers are fostering further innovation, as seen in recent partnerships involving PerkinElmer, Inc.’s IVIS platform. These initiatives are expected to lower user barriers and democratize access to advanced tomographic imaging capabilities.
Looking ahead, the outlook for BTBI remains positive, with ongoing R&D investments focused on expanding detection depth, increasing throughput, and improving molecular specificity. Industry stakeholders anticipate regulatory and workflow standardization to further accelerate adoption across preclinical research and, prospectively, early-stage clinical investigation. As the technology matures, BTBI is poised to become a cornerstone of noninvasive molecular imaging, underpinning critical advances in biomedical science throughout the latter half of the decade.
Technology Overview: Principles of Backscatter Tomographic Bioluminescence Imaging
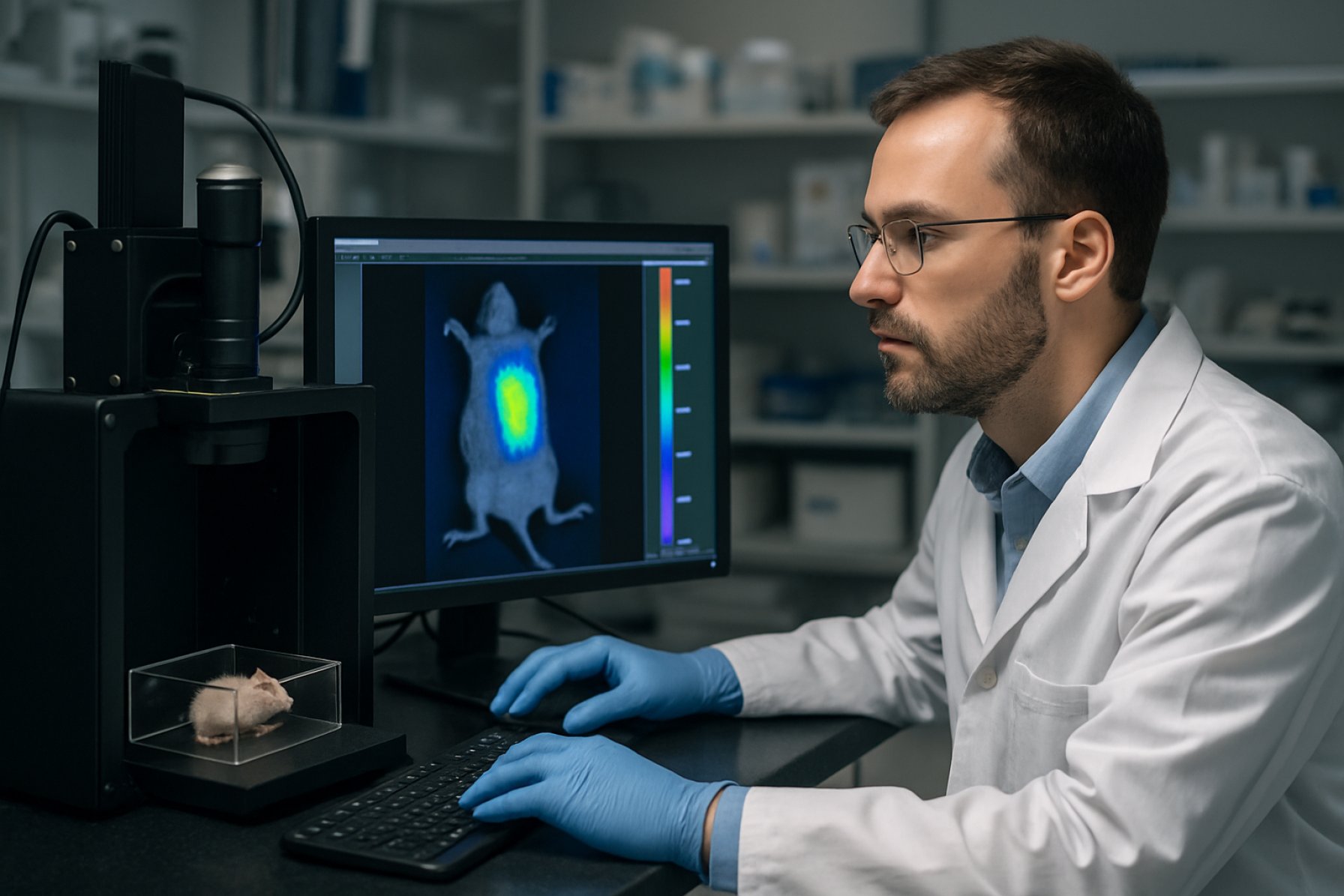
Backscatter Tomographic Bioluminescence Imaging (BTBI) represents a sophisticated evolution of bioluminescence imaging (BLI), designed to overcome the inherent limitations of conventional planar BLI in small animal and preclinical research. While standard BLI provides high sensitivity for monitoring gene expression and cellular events in vivo, its utility is often hampered by poor spatial resolution and limited depth localization, due to photon scattering and absorption in biological tissues. BTBI addresses these challenges by integrating tomographic reconstruction algorithms with advanced photon detection strategies, focusing on the analysis of backscattered light to yield three-dimensional (3D) images of internal bioluminescent sources.
The core principle of BTBI lies in its exploitation of backscattered photons—those emitted from bioluminescent sources within tissue and scattered back toward the surface detectors. By acquiring emission data from multiple surface locations and directions around the subject, BTBI employs computational algorithms to reconstruct the 3D distribution of the bioluminescent signal. This process typically involves solving an inverse problem, using models of light propagation (such as the diffusion approximation to the radiative transfer equation) that account for tissue-specific optical properties.
Recent years have seen rapid progress in the components underpinning BTBI systems. Notably, the introduction of high-sensitivity cooled charge-coupled device (CCD) cameras and complementary metal-oxide-semiconductor (CMOS) detectors has significantly improved the detection of weak bioluminescent signals, even in the presence of considerable background noise. Key manufacturers such as Hamamatsu Photonics and Princeton Instruments continue to advance low-light imaging technologies crucial for BTBI platforms.
On the software side, the integration of robust tomographic reconstruction algorithms—including algebraic reconstruction techniques and maximum likelihood estimation methods—enables more accurate mapping of light sources deep within tissue. Open-source platforms and proprietary software suites developed by leading imaging system suppliers, such as PerkinElmer and Bruker, are facilitating the adoption of BTBI methods in preclinical workflows.
Looking ahead to 2025 and beyond, ongoing research is expected to further enhance BTBI’s spatial resolution and quantitative accuracy by incorporating multispectral imaging, spectral unmixing algorithms, and machine learning-based reconstruction. These advances will likely drive the development of next-generation BTBI instruments, allowing for more precise monitoring of cellular and molecular events in vivo, and accelerating translational research in oncology, neuroscience, and regenerative medicine.
Key Players & Innovators: Leading Companies and Organizations
Backscatter Tomographic Bioluminescence Imaging (BTBI) is an emerging modality in preclinical and potentially clinical imaging, leveraging advances in optical tomography and bioluminescent reporter systems. As of 2025, the field is shaped by a blend of established imaging technology firms, specialized biophotonics companies, and academic innovators translating research breakthroughs into commercial solutions.
- PerkinElmer Inc. remains a dominant force in preclinical optical imaging. Their IVIS Imaging Systems platform is widely used for bioluminescence tomography and has incorporated advanced computational modules to improve 3D reconstruction from backscatter signals. While not branded as “backscatter tomographic,” recent updates point towards more sophisticated light path modeling and photon transport algorithms—key underpinnings for BTBI applications.
- Bruker Corporation is another major player, integrating optical tomography with other modalities such as MRI and PET. Their In-Vivo Xtreme system supports both bioluminescence and fluorescence imaging, and ongoing software upgrades are enhancing tomographic reconstruction and signal backscatter correction, reflecting BTBI’s growing role in multi-modality imaging.
- Photon etc. is advancing imaging hardware with their Phantom platform, focusing on high-sensitivity detection and spectral unmixing. Their development roadmap includes modules for improved depth-resolved imaging, which is critical for accurate BTBI in small animal models and, prospectively, in clinical settings.
- Lightpoint Medical Ltd. is exploring bioluminescent and Cerenkov imaging for surgical guidance. Their LightPath® Imaging System targets intraoperative applications and is adapting tomographic algorithms to exploit backscattered bioluminescent photons, aiming to improve tumor margin detection and procedural outcomes.
- Stanford University and Massachusetts Institute of Technology (MIT) are at the forefront of academic innovation, with several research groups developing open-source BTBI software and prototype imaging devices. Their work is accelerating knowledge transfer to industry, influencing hardware and algorithm design by established players.
Looking ahead, the next few years are expected to see BTBI evolve with deeper integration of artificial intelligence for improved image reconstruction, and wider adoption of hybrid systems combining BTBI with anatomical modalities. Collaborations between academic pioneers and commercial manufacturers will likely drive regulatory approvals and broader clinical translation, consolidating the leadership of these key organizations in the BTBI sector.
Current Applications in Preclinical and Clinical Settings
Backscatter Tomographic Bioluminescence Imaging (BTBI) has emerged as a powerful modality for deep-tissue imaging, particularly within preclinical research settings. In 2025, BTBI is predominantly applied in small animal models for oncology, infectious disease, and gene expression studies. The technology leverages the detection of scattered photons emitted by bioluminescent reporters within biological tissues, enabling noninvasive three-dimensional imaging with high sensitivity and relatively low background signal.
Leading suppliers of preclinical imaging systems, such as PerkinElmer and Bruker, have incorporated advanced bioluminescence tomography capabilities into their platforms. These systems support multi-modal imaging and are frequently used for tracking tumor progression, assessing therapeutic efficacy, and monitoring gene expression in vivo. For example, PerkinElmer’s IVIS Spectrum platform offers features for diffuse tomographic reconstruction, facilitating the quantification and localization of bioluminescent signals in deep tissues.
Recent preclinical studies have demonstrated the utility of BTBI in longitudinal cancer models, where it enables researchers to noninvasively monitor tumor development and response to therapy over extended periods. This capability is instrumental for reducing animal use and improving the statistical power of studies through repeated measurements in the same subjects. BTBI has also been used to visualize infection dynamics and immune cell trafficking in transgenic mouse models expressing bioluminescent reporters.
Clinical translation of bioluminescent imaging faces significant challenges due to the limited tissue penetration of optical photons and the absence of clinically approved bioluminescent reporters for human use. As of 2025, BTBI remains largely confined to preclinical research; however, several academic and industry groups are actively exploring the development of near-infrared (NIR) bioluminescent probes and tomographic algorithms optimized for larger tissue volumes. Purdue University and partners are advancing NIR bioluminescence technology, which could extend the reach of BTBI toward clinical feasibility.
Looking ahead, ongoing improvements in detector sensitivity, tomographic reconstruction algorithms, and the engineering of novel bioluminescent proteins are expected to further enhance the spatial resolution and depth penetration of BTBI. While clinical applications are still on the horizon, the next few years are projected to see expanded use of BTBI in translational research, drug development, and the validation of gene/cell therapies, with instrumentation refinement driven by companies such as PerkinElmer and Bruker.
Market Size, Growth Drivers, and 2025–2030 Forecasts
The market for Backscatter Tomographic Bioluminescence Imaging (BTBI) is expected to see steady growth from 2025 to 2030, driven by advances in preclinical imaging, heightened demand for non-invasive molecular imaging techniques, and increasing investment in translational biomedical research. BTBI technologies, which enable high-sensitivity imaging of bioluminescent signals deep within living tissues, are gaining traction, particularly in oncology, infectious disease research, and drug development.
Current market leaders such as PerkinElmer and Bruker Corporation are expanding their in vivo imaging portfolios with systems that incorporate tomographic reconstruction and enhanced sensitivity to backscatter signals. These improvements allow for more accurate quantification and localization of bioluminescent sources in small-animal models. For example, PerkinElmer’s IVIS line and Bruker Corporation’s In-Vivo Xtreme platform are widely utilized in academic and pharmaceutical research labs, with ongoing upgrades to support advanced applications such as 3D bioluminescence tomography.
Growth is further supported by partnerships between imaging system manufacturers and reagent providers, such as Promega, which supplies luciferase reporter systems tailored for deep-tissue imaging. These collaborations facilitate the development of robust end-to-end solutions, enhancing the reproducibility and throughput of BTBI-based studies.
Looking ahead, the adoption of BTBI is projected to accelerate as researchers prioritize high-content imaging for in vivo cell tracking, gene expression analysis, and immunotherapy monitoring. The emergence of next-generation luciferase substrates and genetically encoded reporters—optimized for red-shifted emission and improved tissue penetration—will further drive the utility and market expansion of BTBI systems (Promega). Furthermore, the integration of artificial intelligence and machine learning algorithms into image reconstruction workflows, as pursued by imaging software developers, is expected to streamline data analysis and interpretation, making BTBI more accessible to a broader user base.
While North America and Europe currently represent the largest markets, significant growth is anticipated in East Asia, particularly in China and Japan, where biomedical research funding and infrastructure development are on the rise (Bruker Corporation). With these drivers in place, the BTBI sector is forecasted to achieve a compound annual growth rate (CAGR) in the high single digits through 2030, propelled by both technological innovation and expanding research applications.
Emerging Trends: AI Integration and Next-Generation Hardware
Backscatter Tomographic Bioluminescence Imaging (BTBI) has evolved rapidly, with 2025 marking a pivotal period characterized by the convergence of artificial intelligence (AI) and advanced hardware design. These trends are reshaping the capabilities and applications of BTBI, especially in preclinical research and translational imaging.
A major development is the integration of AI-driven reconstruction algorithms into BTBI systems. Leading manufacturers such as PerkinElmer and Bruker have announced next-generation imaging platforms that leverage deep learning for improved tomographic reconstructions. AI-based approaches enable enhanced noise reduction, more accurate source localization, and faster image processing, directly addressing longstanding challenges in resolving fine anatomical detail with low photon counts. These advances are particularly relevant for in vivo studies in small animal models, where minimizing acquisition time and maximizing spatial resolution are crucial.
On the hardware front, 2025 has seen the emergence of highly sensitive backscatter detectors and optimized optical components purpose-built for bioluminescence tomography. Hamamatsu Photonics has introduced novel photomultiplier tube (PMT) arrays and silicon photomultiplier (SiPM) modules with enhanced quantum efficiency in the visible to near-infrared range, specifically tailored for bioluminescent signals. These detectors, when coupled with multi-angle gantry systems and adaptive optics, improve photon collection efficiency and enable true 3D tomographic reconstructions, even for deep-seated sources.
Another emerging trend is the adoption of cloud-based data handling and real-time analytics, as highlighted by Miltenyi Biotec, which is piloting integrated cloud computation for high-throughput BTBI workflows. This allows for seamless sharing and collaborative interpretation of large imaging datasets, a feature increasingly demanded in multi-center preclinical trials and drug discovery pipelines.
Looking ahead, the field anticipates further convergence of AI and hardware miniaturization, with several companies pursuing compact, portable BTBI devices for point-of-care and intraoperative applications. The ongoing partnership between Bruker and academic institutions is expected to yield prototype systems with real-time tomographic feedback, accelerating both experimental design and translational research. Furthermore, the adoption of standardized AI training datasets by industry consortia is poised to facilitate cross-platform reproducibility and regulatory acceptance.
Overall, the integration of AI and next-generation hardware is transforming BTBI from a specialized technique to a versatile, high-throughput imaging modality, with significant implications for biomedical research and therapeutic development in the coming years.
Regulatory Landscape and Compliance Updates
Backscatter Tomographic Bioluminescence Imaging (BTBI) is at the forefront of preclinical and translational imaging, offering the potential for high-resolution, deep-tissue visualization using bioluminescent probes. As BTBI technologies mature, regulatory frameworks are evolving to address the unique challenges and safety considerations specific to this modality. In 2025, significant developments are occurring in both the United States and Europe, as regulatory authorities and industry stakeholders seek to harmonize standards and facilitate broader adoption in biomedical research and, potentially, clinical diagnostics.
In the United States, the U.S. Food and Drug Administration (FDA) continues to refine its guidance for preclinical imaging systems, including those leveraging bioluminescence and tomographic reconstruction. Recent FDA communications emphasize the importance of device safety, electromagnetic compatibility, and validation of image reconstruction algorithms for investigational use. BTBI devices intended for animal studies must comply with Good Laboratory Practice (GLP) and Institutional Animal Care and Use Committee (IACUC) protocols. For translational applications, the FDA is reviewing pathways for Investigational Device Exemptions (IDE), focusing on the reproducibility and quantitative accuracy of BTBI systems.
In Europe, the European Medicines Agency (EMA) is collaborating with device manufacturers to ensure that BTBI platforms align with the requirements of the Medical Device Regulation (MDR 2017/745). This regulation, in effect since 2021 but with ongoing implementation updates, mandates comprehensive technical documentation, clinical evaluation, and post-market surveillance for imaging devices. Manufacturers developing BTBI systems must demonstrate conformity with harmonized standards, such as ISO 13485 for quality management and IEC 60601 for electrical safety. The MedTech Europe association has issued sector-specific guidance to assist companies in navigating MDR compliance for novel optical imaging modalities, including BTBI.
Across both regions, there is an increased emphasis on data integrity, cybersecurity, and the interoperability of imaging data, especially as BTBI systems become more integrated with digital health records and analytical platforms. Notably, industry leaders such as PerkinElmer Inc. and Bruker Corporation—who have active portfolios in preclinical optical imaging—are engaging with regulatory bodies to inform standard-setting and validation protocols for BTBI instrumentation.
Looking ahead, the regulatory landscape for BTBI is expected to further clarify, with new guidance anticipated on the clinical translation of bioluminescent tomographic technologies. Stakeholders should monitor updates from the FDA, EMA, and leading industry organizations, as these will shape device development, approval pathways, and global market access for BTBI over the coming years.
Competitive Analysis: Opportunities and Barriers to Entry
Backscatter Tomographic Bioluminescence Imaging (BTBI) is an advanced optical imaging technique that leverages the detection of scattered bioluminescent signals to reconstruct high-resolution tomographic images of biological tissues. As of 2025, several factors define the competitive landscape for companies seeking to enter or expand in this specialized field.
Opportunities:
- Technological Advancements: Recent innovations in low-noise, high-sensitivity cameras and photodetectors, such as those developed by Hamamatsu Photonics and Carl Zeiss AG, have greatly improved BTBI’s sensitivity and spatial resolution. These advances lower the technical entry threshold for new participants who can leverage off-the-shelf components.
- Growing Demand for Non-Invasive Imaging: The increasing requirement for non-invasive and high-throughput preclinical imaging—especially in oncology and neuroscience—creates a strong market pull for BTBI systems. Leading academic users and industry partners are collaborating with system manufacturers, such as PerkinElmer and Bruker Corporation, to develop tailored solutions.
- Open-Source Algorithms and Data: The proliferation of open-source reconstruction algorithms and simulation tools from groups affiliated with institutions like National Institutes of Health and National Cancer Institute offers entrants a springboard for software development, reducing initial R&D investment.
- Regulatory Incentives: In some regions, expedited pathways for preclinical imaging systems—especially those not intended for clinical use—can shorten time-to-market for novel BTBI devices, as evidenced by regulatory guidance from bodies such as the U.S. Food and Drug Administration.
Barriers to Entry:
- Intellectual Property Landscape: Early patent filings by established players, such as PerkinElmer and Bruker Corporation, around proprietary BTBI system components and algorithms may restrict freedom to operate for newcomers.
- Integration Complexity: Effective BTBI requires seamless integration of hardware (optics, cameras) and advanced image reconstruction software. Mastery of both domains is necessary, presenting an interdisciplinary challenge and raising the bar for new entrants.
- Validation and Adoption: Establishing system reliability and reproducibility—especially in comparison with established modalities (e.g., MRI, PET)—is essential for market acceptance. This demands significant investment in validation studies and engagement with key opinion leaders at leading research centers.
- Limited Clinical Application (as of 2025): While BTBI is gaining traction in preclinical research, translation to clinical use faces additional regulatory and technical hurdles, potentially narrowing the initial addressable market.
Outlook: Over the next few years, the BTBI sector is poised for incremental growth, particularly as advances in optics, detectors, and computational imaging reduce system costs and complexity. Strategic collaborations between hardware vendors, academic labs, and biopharma companies are expected to accelerate system adoption and innovation.
Future Outlook: Disruptive Potential Through 2030
Backscatter Tomographic Bioluminescence Imaging (BTBI) is poised to undergo significant advances through 2030, driven by ongoing innovations in photonics, detector sensitivity, and computational tomographic algorithms. By 2025, early prototypes and pilot systems integrating backscatter geometry with tomographic bioluminescence are anticipated to emerge from leading academic and industry collaborations, particularly in preclinical research settings. These systems promise enhanced depth penetration and spatial resolution compared to conventional planar bioluminescence imaging, overcoming a longstanding barrier in the field.
Key photonics companies and scientific instrument manufacturers are expected to play a pivotal role. For example, Hamamatsu Photonics continues to innovate in ultra-sensitive CCD and CMOS sensor arrays, which are central to capturing the faint signals characteristic of deep-tissue bioluminescence with backscatter detection. Similarly, Thorlabs and Carl Zeiss AG have expanded their offerings in high-throughput optics and advanced imaging modules, laying the groundwork for integrated tomographic solutions.
From an applications perspective, BTBI is anticipated to disrupt small animal imaging for oncology and neuroscience. By 2025–2027, several research consortia are projected to validate BTBI for monitoring tumor microenvironments and stem cell dynamics at depths previously inaccessible to non-invasive optical imaging. Partnerships with in vivo imaging solution providers, such as PerkinElmer and Bruker Corporation, are likely to accelerate system prototyping and early adoption, particularly in translational research pipelines.
Computationally, advances in tomographic reconstruction—leveraging AI-driven noise reduction and signal deconvolution—are expected to be commercialized by companies like MathWorks and imaging algorithm specialists collaborating with optical hardware manufacturers. This will further improve the quantitative reliability and clinical potential of BTBI by 2028, facilitating its translation toward early-stage clinical trials.
Looking ahead to 2030, BTBI could become a disruptive force in both preclinical and, prospectively, clinical imaging markets. With the convergence of affordable bioluminescent probes, sensitive backscatter detection hardware, and robust tomographic software, the technology may enable routine, high-resolution imaging of molecular and cellular processes deep within living tissues—heralding a new era in non-invasive diagnostics and therapy monitoring.
References & Official Resources
- PerkinElmer – Manufacturer of IVIS Spectrum and other in vivo imaging platforms with bioluminescence and tomography capabilities.
- Bruker – Developer of the In-Vivo Xtreme and other multimodal optical imaging systems for tomographic bioluminescence studies.
- Molecular Devices – Supplier of in vivo imaging solutions and relevant bioluminescence imaging instrumentation.
- FUJIFILM VisualSonics – Provider of preclinical imaging systems, including those integrating optical and tomographic modalities.
- Charles River Laboratories – Offers contract research and in vivo imaging services, including bioluminescence tomography for drug discovery.
- Microscopy Society of America – Hosts educational resources and technical sessions on advances in optical and tomographic imaging.
- National Institutes of Health (NIH) – Supports research and provides updates on bioluminescence imaging technologies and their applications.