Table of Contents
- Executive Summary: Fungal Genomics QA Market Landscape 2025–2029
- Regulatory Trends and Compliance Standards in Fungal Genomics QA
- Emerging Technologies: AI, Automation, and Next-Gen Sequencing in QA
- Key Industry Players and Strategic Initiatives (2025)
- Market Forecasts: Growth Drivers, Challenges, and Revenue Projections
- Quality Control Protocols: Innovations and Best Practices
- Applications in Agriculture, Healthcare, and Environmental Sectors
- Global Supply Chain and Manufacturing Impacts
- Case Studies: Successful QA Implementation (Official Sources Only)
- Future Outlook: Opportunities, Risks, and Industry Roadmap to 2029
- Sources & References
Executive Summary: Fungal Genomics QA Market Landscape 2025–2029
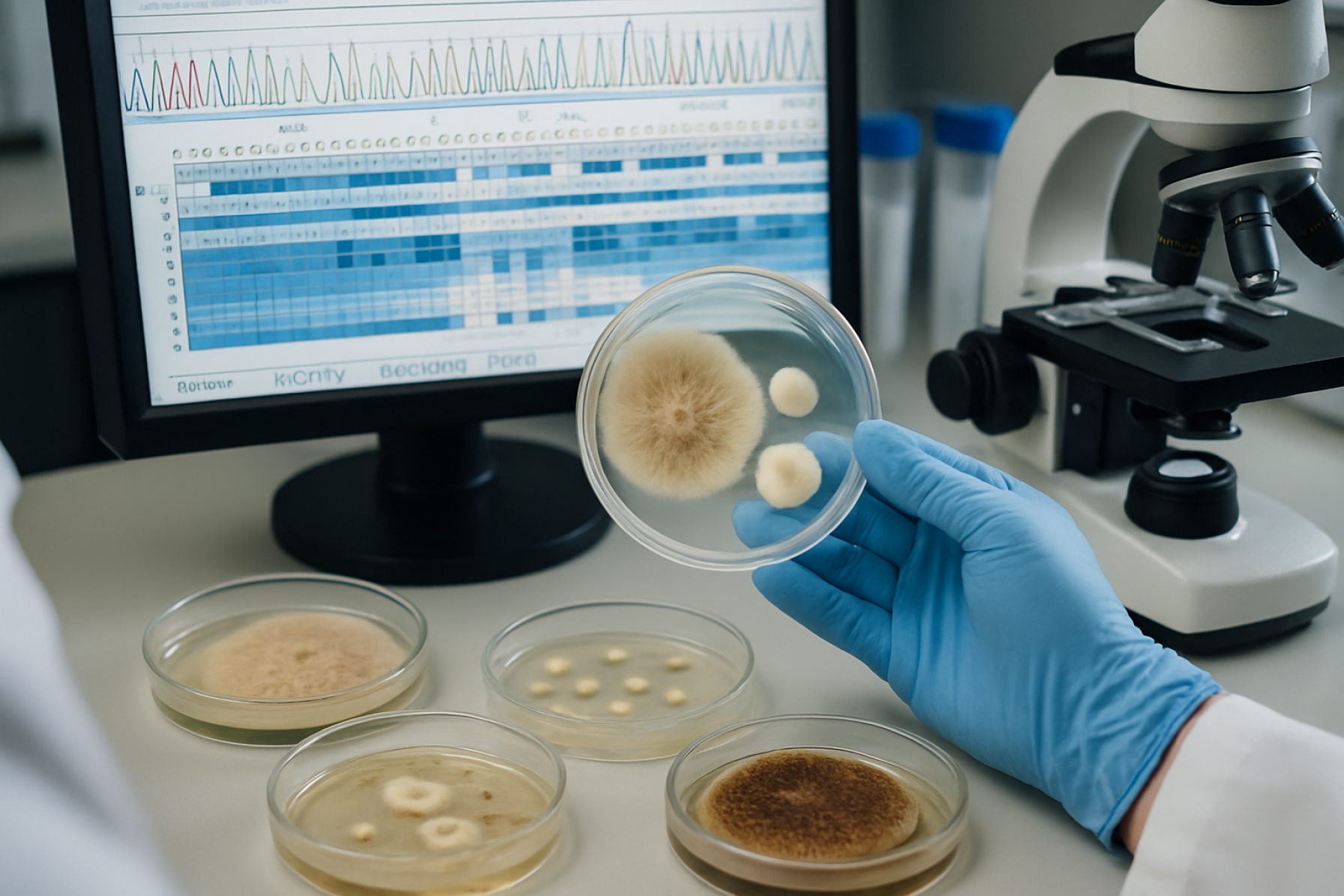
The Fungal Genomics Quality Assurance (QA) market is poised for significant evolution between 2025 and 2029, driven by rapid advances in sequencing technologies, expanding industrial applications, and tightening regulatory standards. As genomics becomes central to sectors ranging from agriculture to pharmaceuticals, robust QA protocols are increasingly essential for accuracy, reproducibility, and regulatory compliance.
In 2025, leading genomics instrumentation providers continue to update their platforms to ensure higher accuracy for fungal genomic applications. For instance, Illumina and Oxford Nanopore Technologies have introduced new reagent kits and software updates specifically tailored for complex fungal genomes, addressing challenges such as high GC content and repetitive regions. These innovations are expected to become industry benchmarks for QA in mycological genomics workflows.
Quality assurance is also being reinforced through the development of standardized reference materials and proficiency testing schemes. The American Type Culture Collection (ATCC) has expanded its catalog of authenticated fungal strains and genomic DNA reference standards, supporting laboratories in validating their sequencing and bioinformatics pipelines. These resources are increasingly adopted by quality-controlled labs as part of accreditation and external quality assessment processes.
On the regulatory front, agencies such as the U.S. Food & Drug Administration (FDA) are collaborating with industry groups to develop guidance on the validation of next-generation sequencing (NGS) for fungal identification and resistance profiling, particularly in clinical and food safety contexts. Such initiatives are expected to shape QA standards globally over the next several years, encouraging the adoption of harmonized protocols and certified workflows.
Meanwhile, bioinformatics solution providers including QIAGEN and Thermo Fisher Scientific are integrating AI-based error correction and contamination detection into their analysis pipelines. These features are designed to enhance QA by automatically flagging potential issues during sequence data processing, further reducing human error and streamlining regulatory compliance.
Looking ahead to 2029, the convergence of automation, regulatory harmonization, and AI-driven analytics is expected to underpin a mature QA landscape in fungal genomics. This will facilitate broader adoption of genomics in diagnostics, food safety, and environmental monitoring, with QA frameworks ensuring data integrity and confidence among stakeholders. The sector will likely see continued collaboration between technology developers, regulatory agencies, and reference standard organizations to align best practices and support the expanding market.
Regulatory Trends and Compliance Standards in Fungal Genomics QA
The regulatory landscape for fungal genomics quality assurance (QA) is experiencing significant evolution in 2025, with a marked emphasis on harmonization and the adoption of advanced molecular standards. Regulatory bodies and standards organizations have recognized the need to address the unique challenges posed by fungal genomics, including high genomic diversity, complex sample matrices, and the critical role of accurate identification in clinical, agricultural, and industrial contexts.
One of the most notable developments is the ongoing update to the International Organization for Standardization’s (ISO) guidelines on next-generation sequencing (NGS) for microbial genomics, which now explicitly includes fungal organisms. The updated ISO 23418:2024 standard outlines requirements for laboratory processes, bioinformatics pipelines, and data interpretation, aiming to improve reproducibility and traceability in fungal genomics workflows. Laboratories worldwide are preparing for compliance audits slated to begin in late 2025, with many investing in staff training and process validation to meet the new benchmarks (International Organization for Standardization).
In parallel, the European Medicines Agency (EMA) has issued guidance for the use of genomic data in the development of antifungal therapeutics. The regulatory framework mandates validated QA procedures for sequencing, annotation, and variant calling, particularly for strains used in clinical trials and reference databases. These measures are designed to mitigate the risk of misidentification or contamination, which can have downstream impacts on drug efficacy and safety assessments (European Medicines Agency).
In the United States, the Food and Drug Administration (FDA) continues to refine its NGS-based microbial identification protocols, with a focus on integrating fungal reference genomes into its regulatory review processes. The agency has partnered with industry and academic stakeholders to expand its curated database of high-quality fungal genomes and to assess the performance of commercially available QA kits (U.S. Food and Drug Administration). Efforts are also underway to align U.S. requirements with international standards, facilitating global trade and data sharing.
Looking ahead, industry groups such as the International Society for Human and Animal Mycology (ISHAM) are working with regulatory authorities to develop sector-specific best practices, particularly in areas like mycotoxin detection and environmental surveillance (International Society for Human and Animal Mycology). These collaborations are expected to yield consensus guidelines by 2026, further strengthening QA frameworks and supporting the safe and effective use of fungal genomics technologies across diverse sectors.
Emerging Technologies: AI, Automation, and Next-Gen Sequencing in QA
In 2025, the integration of artificial intelligence (AI), automation, and next-generation sequencing (NGS) technologies is fundamentally transforming quality assurance (QA) workflows in fungal genomics. Leading sequencing platform providers have introduced advanced instruments with embedded AI-driven analytics, streamlining the detection of sequencing errors, contamination, and variant calling in fungal genomes. For instance, Illumina and Thermo Fisher Scientific have enhanced their NGS systems with machine learning algorithms that assess run quality in real time, flagging anomalies during sequencing and automating corrective actions.
Automation is also advancing sample preparation and data processing. Robotic liquid handling systems, such as those from Beckman Coulter Life Sciences and PerkinElmer, minimize manual error and standardize extraction protocols, ensuring higher reproducibility for critical QA steps. Integration with laboratory information management systems (LIMS) enables complete traceability from sample receipt through analysis, further bolstering compliance with regulatory standards and data integrity requirements.
In the realm of bioinformatics, cloud-based pipelines are being increasingly adopted for QA in fungal genomics. Companies such as DNAnexus and Illumina BaseSpace provide automated platforms where raw sequencing data undergo multiple rounds of quality control, using AI to detect outliers and predict the impact of sequence variation on phenotype and function. This is particularly significant in clinical mycology, where rapid and reliable identification of pathogenic fungi is essential for patient management.
Looking ahead, industry bodies are collaborating to set forth standardized QA benchmarks for fungal genomic data. Organizations like the National Center for Biotechnology Information (NCBI) continue to update their submission protocols and quality metrics for fungal genomes, ensuring harmonization across research and diagnostic laboratories globally. Over the next few years, further convergence of AI, automation, and NGS is anticipated to reduce turnaround times, enhance reproducibility, and enable real-time QA feedback loops, ultimately raising the bar for data quality and reliability in fungal genomics.
Key Industry Players and Strategic Initiatives (2025)
In 2025, quality assurance in fungal genomics is being shaped by a cohort of leading industry players implementing rigorous standards, innovative technologies, and strategic collaborations. As high-throughput sequencing becomes mainstream for fungal identification, diagnostics, and research, the focus on data accuracy, reproducibility, and regulatory compliance is intensifying.
Major sequencing platform manufacturers are advancing quality assurance through integrated controls and software. Illumina has expanded its quality management systems and offers validated workflows specifically for microbial—including fungal—metagenomics. These workflows include built-in controls for contamination, sequence fidelity, and traceability, supporting both clinical and industrial users in meeting regulatory requirements. Similarly, Thermo Fisher Scientific has enhanced its Ion Torrent platforms with new calibration standards and automated reporting features, addressing the demand for traceable and standardized genomic data in mycology laboratories.
Reference database curation and benchmarking remain central to quality assurance. Organizations such as the American Type Culture Collection (ATCC) and National Center for Biotechnology Information (NCBI) are actively updating fungal genome repositories, standardizing metadata, and promoting the use of authenticated strains. This ensures that downstream analyses and diagnostics are grounded in reliable reference materials, reducing errors from misidentification or incomplete genomes.
On the regulatory front, the International Organization for Standardization (ISO) released updated guidelines in late 2024 for molecular diagnostics laboratories, including specifications for fungal genomics workflows (ISO 20387:2024). Industry players are rapidly aligning laboratory practices and software to these standards, with early adopters like SGS and Eurofins Scientific offering ISO-compliant fungal genomics QA services globally.
Strategically, partnerships between sequencing companies, reference repositories, and laboratory accreditation bodies are accelerating. For instance, QIAGEN has launched collaborative programs with clinical and agricultural testing labs to implement end-to-end QA protocols, from sample preservation to data reporting. These alliances aim to streamline compliance and foster best practices as demand for reliable fungal genomics surges in food safety, pharmaceuticals, and environmental monitoring.
Looking ahead, automated QA tools using AI-driven anomaly detection and blockchain-backed data traceability are anticipated from industry leaders by 2026. The ongoing convergence of technology, standards, and industry cooperation is set to further elevate quality assurance benchmarks in fungal genomics worldwide.
Market Forecasts: Growth Drivers, Challenges, and Revenue Projections
The market for Fungal Genomics Quality Assurance is poised for strong growth in 2025 and the ensuing years, driven by expanding applications in agriculture, pharmaceuticals, food safety, and environmental monitoring. With the increasing prevalence of fungal pathogens and the demand for high-quality genomic data, stakeholders are prioritizing robust quality assurance protocols to ensure accuracy, reproducibility, and compliance with regulatory standards.
- Growth Drivers: The adoption of next-generation sequencing (NGS) platforms by research institutions and diagnostic laboratories is a key catalyst. Companies such as Illumina, Inc. and Thermo Fisher Scientific are advancing solutions that incorporate integrated quality control modules for genomic workflows, streamlining the validation of fungal genomes. Additionally, regulatory frameworks, such as those outlined by the U.S. Food and Drug Administration (FDA) for microbial genomics in clinical and food applications, are pushing laboratories to adopt stringent QA practices.
- Challenges: Despite technological advancements, challenges persist. The complexity of fungal genomes—characterized by high variability and repetitive elements—necessitates specialized bioinformatics tools and reference standards for quality benchmarking. Organizations like ATCC are developing authenticated fungal strains and genomic standards to address these hurdles. However, the lack of universally accepted QA protocols and the high costs of implementing comprehensive quality assurance measures may pose barriers for smaller laboratories and emerging markets.
- Revenue Projections: The segment is expected to experience double-digit compound annual growth rates (CAGR) through the late 2020s, with increasing investments from public health agencies and the private sector. Companies offering end-to-end QA solutions for fungal genomics—ranging from sample preparation kits to data analysis software—are projected to capture significant market share. For example, QIAGEN has expanded its molecular quality assurance portfolio to include fungal-specific assays and controls, reflecting rising demand. The integration of automated QA workflows is anticipated to further accelerate market expansion by reducing time-to-result and operational costs.
- Outlook: Looking ahead, the convergence of AI-driven analytics, improved reference databases, and international collaborations—such as those fostered by the World Health Organization (WHO)—will likely standardize QA protocols for fungal genomics on a global scale. These trends are expected to open new revenue streams and enhance data reliability, underpinning the sector’s robust outlook through 2025 and beyond.
Quality Control Protocols: Innovations and Best Practices
In 2025, quality assurance in fungal genomics is rapidly advancing, propelled by innovations in sequencing technology, data analysis platforms, and standardized protocols. Accurate identification and functional analysis of fungal genomes require rigorous quality control (QC) throughout the workflow, from sample collection to bioinformatic interpretation. Key industry leaders and research organizations are spearheading initiatives to standardize and improve QC protocols, ensuring data reliability and reproducibility across laboratories.
One significant trend is the integration of automated QC checks within next-generation sequencing (NGS) instruments. Manufacturers such as Illumina and Thermo Fisher Scientific have embedded real-time quality metrics—such as base calling accuracy, library complexity, and contamination detection—into their sequencing workflows. These automated systems minimize human error and enable prompt remediation when quality thresholds are not met.
Industry-wide efforts to harmonize fungal genomics QC are evident in initiatives like the development of standardized reference materials. The National Institute of Standards and Technology (NIST) is actively working on microbial genomic standards, including fungal species, to provide laboratories with quality benchmarks for sequencing accuracy, contamination monitoring, and variant calling. These reference materials are pivotal for proficiency testing and inter-laboratory comparisons.
Best practices are also evolving in sample preparation and library construction. Companies such as New England Biolabs and QIAGEN have released robust kits and protocols optimized for challenging fungal samples, reducing biases and improving reproducibility. Their protocols emphasize stringent controls for DNA purity, fragmentation, and adapter ligation, which are critical for minimizing artifacts in downstream analysis.
On the bioinformatics front, platforms like NCBI and EMBL-EBI are increasingly providing standardized pipelines and annotation tools tailored to fungal genomes. These resources incorporate built-in QC modules—such as sequence quality assessment, contamination screening, and assembly validation—enabling users to flag and correct anomalies before publication or downstream application.
Looking ahead, the adoption of artificial intelligence (AI)-driven QC, multi-omics integration, and real-time monitoring tools is anticipated to further elevate the rigor and efficiency of fungal genomics quality assurance. Industry collaborations and public-private partnerships are expected to accelerate the dissemination of best practices and the establishment of universal standards, fostering greater confidence in fungal genomics data for clinical, agricultural, and environmental applications.
Applications in Agriculture, Healthcare, and Environmental Sectors
In 2025, the assurance of quality in fungal genomics is gaining heightened attention across agriculture, healthcare, and environmental sectors. As high-throughput sequencing platforms become more affordable and accessible, the demand for reliable and reproducible genomic data is driving major industry and academic initiatives to set and enforce quality standards.
In agriculture, fungal genomics underpins efforts to breed disease-resistant crops and monitor plant pathogens. Leading agricultural genomics solution providers are deploying robust quality management systems to ensure the fidelity and traceability of sequencing data. For example, Illumina, Inc. continues to refine its sample preparation and data validation protocols, integrating automated quality checks and offering standardized workflows for plant pathogen genomics. These measures are designed to minimize errors in detecting virulent fungal strains that threaten global food security.
The healthcare sector, particularly in fungal infection diagnostics and antifungal drug development, is witnessing the integration of certified reference materials and external proficiency testing schemes. Organizations such as Thermo Fisher Scientific provide validated kits and bioinformatics pipelines that comply with regulatory standards for clinical genomics laboratories. This reduces the risks of false-positive or false-negative results in the identification of clinically relevant fungi, ensuring patient safety and supporting the development of targeted therapies.
Environmental monitoring initiatives are increasingly reliant on metagenomic surveys to assess fungal biodiversity and ecosystem health. In 2025, collaborative quality assurance frameworks are emerging, such as those facilitated by the DOE Joint Genome Institute, which provides reference genomes and standardized protocols for environmental sequencing projects. These frameworks address challenges related to sample contamination, data normalization, and annotation accuracy—critical for biodiversity conservation and bioremediation efforts.
Looking ahead to the next few years, industry consortia and standards bodies are expected to further harmonize quality benchmarks. The adoption of automated sample tracking, machine learning-based anomaly detection, and international proficiency schemes is anticipated to become standard practice. Companies like Pacific Biosciences are also advancing long-read sequencing technologies that offer higher accuracy for complex fungal genomes, which will require updated quality metrics and controls.
Overall, the coming years will see fungal genomics quality assurance become a cornerstone for reliable applications in agriculture, healthcare, and environmental sectors, reinforced by cross-sector collaborations and technological innovation.
Global Supply Chain and Manufacturing Impacts
The global supply chain and manufacturing landscape for fungal genomics is undergoing significant transformation in 2025, driven by increasing demand for high-quality, reproducible genomic data and the need to ensure traceability across international networks. As fungal genomics finds broader application in areas such as agriculture, pharmaceuticals, biotechnology, and food safety, robust quality assurance (QA) frameworks are becoming a central focus for both suppliers and end-users.
A notable trend is the implementation of harmonized quality management systems (QMS) in sequencing facilities and reagent manufacturing. Major sequencing platform providers, such as Illumina, Inc. and Thermo Fisher Scientific, have enhanced their quality control protocols in line with ISO 9001:2015 and ISO 13485:2016, aiming to ensure batch-to-batch consistency and data integrity in fungal genomics workflows. These standards are increasingly required by multinational clients and regulatory agencies, influencing suppliers across North America, Europe, and Asia-Pacific.
In 2025, global supply chains for critical reagents—such as DNA extraction kits, PCR enzymes, and library preparation chemicals—are benefitting from the establishment of regional manufacturing hubs. Companies like QIAGEN have expanded their local production and QA testing in strategic locations to mitigate risks of raw material shortages and shipping delays, as experienced during the COVID-19 pandemic. This shift supports more resilient, transparent supply chains and enables faster response to quality incidents.
There is also accelerated adoption of digital QA systems, leveraging cloud-based platforms for real-time lot tracking, certificate management, and automated audit trails. Life Technologies Corporation (part of Thermo Fisher Scientific) has integrated these digital QA tools to streamline regulatory compliance and provide customers with up-to-date quality documentation for genomic products. Blockchain technology pilots for tracking the provenance of critical consumables are underway, aimed at curbing counterfeiting and ensuring traceability from manufacturer to lab bench.
Looking forward, collaborative efforts among suppliers, sequencing service providers, and regulatory bodies such as the International Organization for Standardization (ISO) are expected to yield sector-specific QA guidelines for fungal genomics by 2027. These initiatives will likely incorporate requirements for reproducibility, contamination control, and standardized reference materials. As a result, supply chains will see further strengthening of quality checkpoints, improved interoperability among data systems, and a higher overall standard for fungal genomics products worldwide.
Case Studies: Successful QA Implementation (Official Sources Only)
Quality assurance (QA) in fungal genomics is increasingly recognized as a cornerstone for reliable research, diagnostics, and industrial application. In 2025, several notable organizations have showcased successful QA implementation, demonstrating best practices and tangible benefits across diverse use cases.
-
Public Health England (PHE): Standardization in Fungal Pathogen Genomics
As part of its national reference laboratory services, UK Health Security Agency (formerly PHE) has implemented a rigorous QA framework for whole-genome sequencing (WGS) of fungal pathogens. Their protocols entail routine use of reference strains, proficiency testing, and participation in international ring trials. In 2024–2025, these measures contributed to high-confidence identification of Candida auris outbreaks in UK hospitals, with genome data consistently validated across multiple platforms and laboratories. -
US Centers for Disease Control and Prevention (CDC): External Quality Assessment (EQA) Panels
The Centers for Disease Control and Prevention has led external quality assessment programs for laboratories performing fungal WGS, particularly for surveillance of emerging antifungal resistance. In 2023–2025, CDC distributed blinded EQA panels containing DNA from clinically significant fungi. The results informed improvements in sample processing, sequence quality metrics, and bioinformatics pipeline validation, with participant labs reporting increased accuracy and reproducibility. -
Illumina: End-to-End Quality Metrics in Sequencing Workflows
Illumina, a global leader in sequencing technology, has highlighted case studies from 2024–2025 in which fungal genomics labs adopted its BaseSpace Sequence Hub and integrated quality control checkpoints. These include real-time monitoring of sequencing run metrics, automated contamination detection, and standardized variant calling. Labs using these solutions reported a decrease in failed runs and enhanced confidence in downstream analyses for industrial biotechnology projects. -
European Molecular Biology Laboratory (EMBL): Accredited Reference Genomes
The European Molecular Biology Laboratory has played a pivotal role in establishing reference genomes and curated databases for fungi, with strict QA criteria for genome assembly and annotation. Ongoing work in 2025 emphasizes transparent benchmarking and reproducibility, supporting both research consortia and commercial partners in ensuring the reliability of genomic resources.
These case studies illustrate that successful QA in fungal genomics hinges on standardized protocols, external assessments, robust technology platforms, and international collaboration. As sequencing technologies and applications continue to evolve, such QA frameworks are expected to become even more integral to trustworthy outcomes across clinical, environmental, and industrial domains.
Future Outlook: Opportunities, Risks, and Industry Roadmap to 2029
As fungal genomics becomes increasingly integrated into pharmaceutical development, agriculture, and environmental monitoring, the focus on quality assurance (QA) is intensifying. By 2025, advances in high-throughput sequencing and bioinformatics are driving rigorous QA protocols to ensure data reliability, reproducibility, and regulatory compliance. Companies and organizations are spearheading efforts to standardize methods, control contamination, and validate both wet-lab and computational pipelines.
Looking ahead to 2029, several opportunities and risks are emerging. The proliferation of third-generation sequencing platforms, such as those from Oxford Nanopore Technologies and Pacific Biosciences, is providing longer reads and improved accuracy, enabling deeper insights into complex fungal genomes. However, these technological advances necessitate more sophisticated QA strategies, particularly regarding error correction, reference genome selection, and data interoperability.
Industry bodies including the European Bioinformatics Institute (EMBL-EBI) and the National Center for Biotechnology Information (NCBI) are expanding their best-practice guidelines and quality benchmarks to address the unique challenges of fungal genomics, such as high rates of horizontal gene transfer and structural genome variation. These organizations are also working towards globally harmonized data submission standards and curated reference databases, which are essential for large-scale comparative studies and regulatory submissions.
- Opportunities: Automation and AI-driven quality control tools are emerging, with companies like Illumina and Thermo Fisher Scientific investing in software that flags anomalies, tracks provenance, and streamlines validation. These technologies are expected to reduce manual QA bottlenecks and enable real-time monitoring of sequencing runs.
- Risks: As data volumes surge, the risk of cross-contamination, misannotation, and cyber threats to genomic databases increases. The industry must continuously update protocols for sample handling, chain-of-custody documentation, and cybersecurity to safeguard data integrity and patient privacy.
- Roadmap: By 2029, industry stakeholders aim to establish end-to-end QA frameworks integrating biobanking, sequencing, and informatics. Collaboration between technology providers, regulatory agencies, and international consortia will be essential in defining threshold criteria for genome assembly quality, variant calling accuracy, and metadata completeness.
Overall, the next five years are poised to see fungal genomics QA evolve from fragmented, lab-specific practices to robust, industry-wide systems, ensuring that genomic data can be confidently leveraged for diagnostics, therapeutics, and biosecurity applications.
Sources & References
- Illumina
- Oxford Nanopore Technologies
- American Type Culture Collection (ATCC)
- QIAGEN
- Thermo Fisher Scientific
- International Organization for Standardization
- European Medicines Agency
- International Society for Human and Animal Mycology
- PerkinElmer
- DNAnexus
- Illumina BaseSpace
- National Center for Biotechnology Information (NCBI)
- SGS
- World Health Organization (WHO)
- National Institute of Standards and Technology (NIST)
- EMBL-EBI
- Life Technologies Corporation
- UK Health Security Agency
- Centers for Disease Control and Prevention
- European Molecular Biology Laboratory